Global Infectious Disease Diagnostics Market Analysis and Insights:
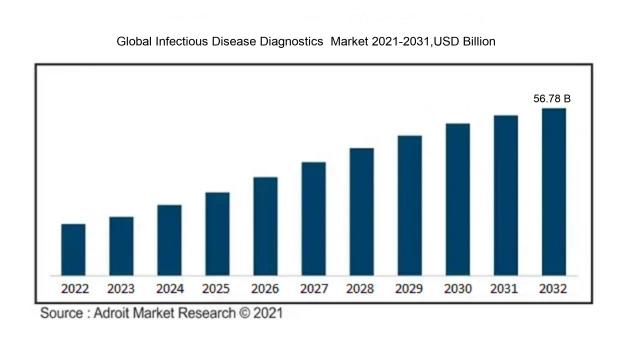
In 2023, the size of the worldwide Infectious Disease Diagnostics market was US$ 28.62 billion. Adroit Market Research projects that the market will increase at a compound annual growth rate (CAGR) of 7.5 % from 2024 to 2032, reaching US$ 56.78 billion.
The market for infectious disease diagnostics experiences robust growth driven by several significant factors. A notable increase in the prevalence of infectious conditions, including HIV, tuberculosis, and hepatitis, necessitates precise and prompt diagnostic solutions. Advances in technology—such as polymerase chain reaction (PCR), next-generation sequencing, and point-of-care diagnostics—are improving both the speed and accuracy of disease identification. Furthermore, ened investments from both governmental bodies and the private sector in healthcare and diagnostic innovations are promoting development and accessibility. The global awareness around infectious diseases, amplified by health emergencies like the COVID-19 pandemic, has also led to a surge in demand for diagnostic tools. Additionally, rising healthcare budgets, the proliferation of diagnostic laboratories, and continuous research endeavors aimed at developing new diagnostic methodologies significantly support the expansion of this market. Lastly, the urgent need for swift diagnostic tests that facilitate clinical decision-making and infection management further drives favorable market trends.
Infectious Disease Diagnostics Market Definition
Infectious disease diagnostics encompasses the various techniques and procedures employed to pinpoint the specific pathogens responsible for infections in patients. This field involves a range of laboratory evaluations and technological approaches aimed at identifying the existence of bacteria, viruses, fungi, or parasites within clinical specimens.
The diagnosis of infectious diseases plays a pivotal role in the swift identification and management of illnesses, facilitating effective treatment and outbreak containment. Precise diagnostic methods are essential for distinguishing among different pathogens, assisting healthcare providers in choosing suitable therapies while reducing the likelihood of unnecessary prescriptions, thus aiding in the fight against antibiotic resistance. Moreover, early identification of infections significantly improves patient outcomes and curtails their transmission within communities. Diagnostic surveillance is instrumental in supporting public health measures by monitoring disease patterns and shaping vaccination policies. In summary, dependable diagnostic tools are vital for protecting global health, enabling quick responses to new infectious challenges, and optimizing healthcare resource distribution.
Infectious Disease Diagnostics Market Segmental Analysis:

Insights On Key Product
Reagents
Reagents are expected to dominate the Global Infectious Disease Diagnostics Market due to their critical role in various diagnostic procedures. These are essential components in test kits and laboratory processes, helping to accurately identify pathogens in samples. Given the increasing prevalence of infectious diseases worldwide and the rising demand for rapid and accurate diagnostic solutions, the market for reagents is growing significantly. The ongoing developments in biotechnology and the trend toward personalized medicine further amplify the demand for high-quality reagents, making them a leading product category in this market.
Instruments
Instruments play a vital role in the diagnostics process, providing the necessary technology for conducting tests and analyzing results. Devices such as PCR machines, immunoassay analyzers, and sequencers are crucial for timely diagnostics. The continuous advancements and upgrades in diagnostic instruments, along with increased healthcare investment globally, contribute to the growth in this sector. However, while they remain important, they do not match the rising demand for reagents.
Kits & Consumables
Kits and consumables are integral to the diagnostics process, particularly as they combine reagents and tools needed for testing. The convenience of all-in-one kits for various infectious diseases makes them popular among healthcare providers. Yet, the market growth is primarily driven by the increasing need for reagents, which are fundamental components of these kits. While kits have their importance, they significantly rely on the superior demand for quality reagents.
Services
Services associated with diagnostics, such as testing, reporting, and consultation, are essential for effective disease management. This area ensures that healthcare providers can offer rapid and reliable results to patients. However, the services sector tends to follow the product-centric areas like reagents or instruments, as the accuracy and efficiency of testing are ultimately reliant on these products. Thus, while services are crucial, they do not dominate the market as reagents do.
Insights On Key Technology
Immunodiagnostics
Immunodiagnostics is anticipated to dominate the Global Infectious Disease Diagnostics market. It is a critical approach that utilizes antibody-antigen interactions for detecting infectious agents, marking its significance in serial testing and screening. This technology encompasses various methods such as enzyme-linked immunosorbent assays (ELISA) and rapid diagnostic tests (RDTs). While it may lack the molecular specificity of methods like PCR, immunodiagnostics offers the advantage of detecting infections based on immune responses and is often used for diseases where antibodies can be detected more quickly than the pathogen itself. Its established presence in clinical laboratories underscores its role in infectious disease monitoring and control.
Polymerase Chain Reaction
The Polymerase Chain Reaction (PCR) technology is growing rapidly due to its unmatched speed, sensitivity, and specificity in detecting various pathogens. PCR has revolutionized molecular diagnostics by allowing rapid amplification of DNA, thus enabling the timely identification of infectious diseases, which is critical for effective treatment. Its applications span clinical laboratories, research institutes, and even remote settings, aided by advancements such as Real-Time PCR and digital PCR technologies. Additionally, the ongoing development of point-of-care PCR testing is improving accessibility and convenience, further solidifying its leading position in the market.
Clinical Microbiology
Clinical Microbiology remains a vital component of infectious disease diagnostics. This technology focuses on isolating and identifying pathogens from clinical specimens through culture and biochemical tests. Its importance lies in the ability to provide comprehensive candid results regarding bacterial, viral, and fungal infections. Although it is time-consuming compared to molecular methods, it offers the advantage of identifying antibiotic susceptibility, critical in guiding treatment. The established frameworks and methodologies in clinical microbiology will continue to support its sustenance in the market, especially in traditional healthcare settings.
In Situ Hybridization
In Situ Hybridization (ISH) is an important diagnostic tool utilized primarily for the localization of specific nucleic acid targets in tissue samples. It offers unique advantages, such as allowing for the observation of gene expression and chromosomal abnormalities in their cellular context. Although its application is more niche compared to broader techniques, ISH plays a crucial role in cancer diagnosis and research on infectious diseases caused by specific pathogens. Advances in fluorescent ISH and its combination with other techniques are likely to enhance its relevance in pathology and infectious disease diagnostics.
Isothermal Nucleic Acid Amplification Technology
Isothermal Nucleic Acid Amplification Technology (INAAT) is gaining traction due to its ability to amplify nucleic acids at a constant temperature without the need for thermal cycling. This feature simplifies testing procedures, making it cost-effective and suitable for point-of-care applications. Companies are increasingly adopting INAAT for diagnosing various infectious diseases since it can provide results in a matter of minutes, rather than hours. Despite its smaller share in the market, ongoing innovations and the increasing demand for quick diagnostic tests reinforce the potential growth of INAAT in the infectious disease landscape.
Insights On Key Application
Respiratory Diseases
Respiratory diseases constitute a substantial portion of the infectious disease diagnostics market and is expected to dominate the market, largely due to the diverse array of viruses and bacteria affecting this system. Conditions such as influenza, pneumonia, and bronchitis are widespread, resulting in increased healthcare visits and diagnostic testing. The ongoing evolution of testing methods—like multiplex PCR that can identify multiple pathogens simultaneously—enhances the accuracy and efficiency of diagnoses. This expansion boosts demand across healthcare settings, particularly in the wake of increased awareness of respiratory infections post-pandemic, positioning this category as a significant market player.
COVID-19
The COVID-19 application area is expected to grow significantly due to the ongoing repercussions of the pandemic. Compared to other infectious diseases, COVID-19 diagnostics have rapidly evolved, leading the market in innovative testing technologies and methodologies like PCR, antigen tests, and serological testing. The ened global focus, due to various waves of infections and new variants, along with government mandates for testing, has propelled investments and advancements in diagnostic solutions for this virus. As healthcare systems continue to prioritize efficient and accurate COVID-19 testing, it is anticipated that this category will maintain a leading position in market revenue and growth.
Gastrointestinal Tract Infections
Gastrointestinal tract infections are a critical area within the infectious disease diagnostics market. The prevalence of pathogens such as Salmonella and E. coli continues to affect millions globally, leading to a significant demand for rapid and accurate diagnostic tests. Increased awareness of foodborne illnesses and enhanced food safety regulations are pushing both healthcare providers and industries to seek reliable testing methods. Moreover, research and development in novel diagnostic technologies are expected to facilitate timely diagnosis, thereby improving treatment outcomes and driving the market's growth in this category.
Sexually Transmitted Infection
Sexually transmitted infections (STIs) play a notable role in the infectious disease diagnostics domain. With rising instances of infections such as chlamydia, gonorrhea, and syphilis, diagnosis is crucial for effective treatment and prevention strategies. The market is seeing advancements in rapid testing kits and point-of-care testing methods that facilitate immediate diagnosis, particularly among high-risk groups. The increasing emphasis on sexual health education and awareness programs is further bolstering this, leading to a notable demand for innovative diagnostic tools designed to combat STIs.
Tuberculosis
Tuberculosis (TB) remains a significant focus in the infectious disease diagnostics market due to its high mortality rate and global prevalence, especially in developing countries. As TB is often undiagnosed because of its complex nature, robust diagnostic solutions such as GeneXpert and other molecular tests are gaining traction to ensure early detection and treatment. Efforts from global health organizations to eliminate TB further amplify the need for effective diagnostic methods. Continued funding and research in this area are set to propel advancements and ultimately enhance diagnostics related to tuberculosis.
Hospital-acquired Infection
Hospital-acquired infections (HAIs) represent a crucial in the infectious disease diagnostics space. As infections like MRSA and C. difficile pose significant risks to patient safety, hospitals are increasingly focusing on diagnostic solutions that can reduce infection rates. The adoption of rapid and accurate testing methods is progressing alongside strict regulatory frameworks aimed at improving patient outcomes in healthcare settings. This focus on infection control and prevention measures ensures a growing demand for diagnostics related to hospital-acquired infections, solidifying its importance in the broader market landscape.
Mosquito-borne Diseases
Mosquito-borne diseases such as dengue, malaria, and Zika virus continue to underscore the importance of diagnostics in infectious disease management. The geographical expansion of vector-borne diseases due to climate change is driving the need for reliable and rapid diagnostic tools. Emerging technologies offer innovative solutions for the rapid identification of these diseases in endemic regions, thus improving patient management and outbreak control. Government initiatives, alongside global health efforts, are committed to tackling these diseases, thereby positively impacting the demand for effective diagnostic methods in this category.
Insights On Key End-use
Hospitals
The Hospitals category is expected to dominate the Global Infectious Disease Diagnostics Market due to their centralized role in healthcare and patient management. Hospitals are increasingly adopting advanced diagnostic technologies to provide timely treatment, especially during infectious disease outbreaks. With the ongoing demand for rapid diagnostic tools, hospitals leverage comprehensive testing capabilities to diagnose and treat infections effectively. Additionally, hospitals often have better funding and resources to invest in innovative diagnostic techniques, contributing to a growing market share. Their critical importance in addressing public health challenges, combined with technological advancements, positions hospitals as the frontrunner in this market.
Clinics
Clinics play a vital role in the infectious disease diagnostics market, especially with the rising trend of outpatient services. They provide accessible healthcare to the local population and often act as the first point of contact for patients exhibiting infectious disease symptoms. The convenience of rapid testing and results offered by clinics enhances patient satisfaction. Moreover, the growing emphasis on preventive care has led clinics to invest in diagnostic tools, promoting early detection and intervention, which ultimately reduces the burden on hospitals.
Diagnostics Laboratories
Diagnostics Laboratories are crucial in the infectious disease diagnostics landscape, as they specialize in testing and analyzing samples to diagnose diseases accurately. Their continuous innovations in testing methods and technologies adapt to emerging pathogens and epidemics, ensuring reliable results. Many laboratories partner with healthcare facilities to provide extensive support for complex cases. The increasing need for accurate diagnostics in public health initiatives and research drives the growth of this, making them an integral part of the overall diagnostics ecosystem.
Research Institutes
Research Institutes are significant contributors to the infectious disease diagnostics market, driving innovation through research and development. Their focus on discovering new pathogens and developing advanced diagnostic techniques enhances the overall understanding of infectious diseases. These institutes foster collaboration with healthcare professionals and industry players, promoting advancements in diagnostics. Although their impact may be more indirect compared to hospitals and clinics, their foundational role in technology and method development in diagnostics lays the groundwork for future market growth.
Others
The 'Others' category encompasses various entities that do not fit neatly into the major s of hospitals, clinics, diagnostics laboratories, and research institutes. This may include home testing kits, telemedicine services, or even public health organizations focused on infectious disease management. While their share of the market might be smaller, their significance is growing due to the increasing reliance on technology and home-based testing solutions. The rising consumer preference for convenience and immediate access to diagnostic tools is likely to enhance the growth prospects within this category as healthcare evolves.
Global Infectious Disease Diagnostics Market Regional Insights:
North America
North America is expected to dominate the Global Infectious Disease Diagnostics market due to a combination of factors including advanced healthcare infrastructure, robust research and development capabilities, and a high prevalence of infectious diseases. The region benefits from significant investment in diagnostic technologies and innovation, with major players located in the United States driving the market forward. Furthermore, the increasing trend of point-of-care testing and ened awareness around infectious diseases, particularly in light of recent global pandemics, has fueled demand. Supportive government initiatives aimed at improving healthcare access also bolster the region's position, making it the leading market for infectious disease diagnostics.
Latin America
Latin America is characterized by a growing healthcare sector that is gradually adopting innovative diagnostic solutions. Although it does not lead the market as North America does, the region is witnessing an increase in infectious disease incidences, which drives demand for better diagnostic tools. Economic growth is leading to enhanced healthcare spending, facilitating the adoption of advanced diagnostics. Moreover, partnerships between local companies and international manufacturers are helping to improve access to essential diagnostic services and technologies.
Asia Pacific
The Asia Pacific region shows significant potential in the Infectious Disease Diagnostics market, largely driven by its vast population and rising burden of infectious diseases. Countries like India and China are experiencing rapid growth in healthcare infrastructure, leading to increased demand for diagnostics. However, challenges such as regulatory hurdles and varying levels of healthcare access may hinder rapid advancement. Nevertheless, with a strong emphasis on preventive healthcare and investments in technological advancements, the region could become a key player in the future of infectious disease diagnostics.
Europe
Europe is a robust market for infectious disease diagnostics, characterized by high standards of healthcare and significant investment in medical research. The region benefits from strong regulatory frameworks and collaborative efforts between public and private sectors. While it faces competition from North America, Europe is making strides in developing advanced diagnostic technologies and promoting regional public health initiatives. The increasing incidence of multi-drug resistant infections and a proactive approach to healthcare are propelling the region’s demand for precise and efficient diagnostic tools, ensuring its relevance in the sector.
Middle East & Africa
The Middle East & Africa region is gradually making progress in the infectious disease diagnostics market. While facing challenges such as limited healthcare budgets and infrastructure, there is a noticeable increase in investment from both local and international entities aiming to improve healthcare access. The rising burden of infectious diseases like tuberculosis and HIV/AIDS is pushing governments and organizations to enhance diagnostic capacities. Innovative supply chain solutions and partnerships in the region could lead to improved access and adoption of diagnostic technologies, ultimately driving growth in this market.
Infectious Disease Diagnostics Market Competitive Landscape:
Major contributors in the global infectious disease diagnostics sector play a crucial role in advancing cutting-edge diagnostic technologies that facilitate swift and precise disease identification. Their joint initiatives in research, development, and distribution greatly improve the management and monitoring of diseases on a worldwide scale.
Prominent companies in the market for Infectious Disease Diagnostics consist of Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, Hologic, BioMérieux, Cepheid, QIAGEN, Becton, Dickinson and Company, Danaher Corporation, PerkinElmer, Illumina, Meridien Bioscience, Agilent Technologies, and Luminex Corporation.
Global Infectious Disease Diagnostics Market COVID-19 Impact and Market Status:
The COVID-19 pandemic has notably expedited the expansion of the worldwide market for infectious disease diagnostics, driven by a surge in the need for quick testing solutions and advanced diagnostic methodologies.
The COVID-19 pandemic brought about profound changes in the market for infectious disease diagnostics, spurring both growth and innovation. The pressing demand for swift and precise testing catalyzed increased funding for various diagnostic technologies, such as PCR, antigen, and serological assays. This ened need led to a wider adoption of point-of-care testing, which improved both accessibility and speed for disease identification. Furthermore, the pandemic underscored the vital role of diagnostics in public health initiatives, encouraging partnerships among businesses, governmental entities, and healthcare organizations to strengthen diagnostic capabilities. In response, numerous firms in the diagnostics field expanded their offerings and streamlined regulatory pathways to accelerate the development of testing solutions. The trend toward multiplex testing, enabling the concurrent identification of multiple pathogens, also gained traction, influencing future directions within the industry. Ultimately, the pandemic not only intensified competition but also emphasized the necessity for technological innovation and readiness for potential future outbreaks of infectious diseases.
Latest Trends and Innovation in The Global Infectious Disease Diagnostics Market:
- In February 2023, Abbott announced the launch of its ID NOW COVID-19 2 test, which offers rapid molecular testing for the detection of SARS-CoV-2 with results available in just 13 minutes, highlighting significant advancements in near-patient testing technology.
- In September 2022, Roche completed the acquisition of TIB Molbiol, a company specializing in the development of molecular assays for infectious diseases. This acquisition strengthens Roche's capabilities in the molecular diagnostics space, particularly for respiratory pathogens.
- In June 2023, Thermo Fisher Scientific launched the QuantStudio 7 Pro Digital PCR System, enhancing the detection and quantification of pathogens and enabling precise infectious disease diagnostics, which is crucial for timely treatment decisions.
- In March 2023, Cepheid received Emergency Use Authorization from the U.S. FDA for its Xpert Xpress SARS-CoV-2/Flu/RSV test, which simultaneously detects COVID-19, influenza A, influenza B, and respiratory syncytial virus (RSV) in a single sample, demonstrating innovation in multiplex testing capabilities.
- In December 2022, Hologic announced its acquisition of Biotheranostics, which enhances Hologic's diagnostic portfolio with innovative tests for infectious diseases, thereby expanding its market reach and service offerings.
- In October 2022, Becton, Dickinson and Company (BD) launched its BD MAX™ System for molecular diagnostics, designed to rapidly and accurately test for various infectious diseases, demonstrating a commitment to advancing diagnostic technologies.
- In April 2023, Siemens Healthineers unveiled advancements in its Atellica® Solution, integrating new assays for infectious disease testing that improve automation and laboratory efficiency.
- In January 2023, BioMérieux announced the launch of its NEXTGEN platform, focusing on significantly reducing time to diagnosis for infections and providing advanced solutions for clinicians managing infectious diseases.
Infectious Disease Diagnostics Market Growth Factors:
The expansion of the Infectious Disease Diagnostics Market is propelled by technological innovations, a surge in the incidence of infectious diseases, and a ened need for swift and precise diagnostic solutions.
The Infectious Disease Diagnostics Market is experiencing substantial growth driven by several key factors. A rise in the global incidence of infectious diseases highlights the urgent requirement for effective detection and monitoring solutions. The emergence of new pathogens, including COVID-19 and antibiotic-resistant strains, has increased the need for swift and precise diagnostic testing. Innovations in technology, especially in areas like molecular diagnostics and point-of-care testing, have improved the speed and accuracy of disease detection, attracting healthcare providers focused on enhancing efficiency.
Moreover, growing healthcare budgets and governmental efforts aimed at strengthening public health frameworks significantly contribute to the expansion of this market. The rising trend towards personalized medicine is also supportive of the development of specialized diagnostic tests tailored to individual patient needs. Increased awareness regarding the advantages of early detection, along with a ened emphasis on preventive care, is driving demand for cutting-edge diagnostic solutions.
Additionally, strategic partnerships between diagnostic firms and healthcare institutions, along with increased investment in research and development for advanced diagnostic techniques, are expected to enhance market dynamics. Together, these elements foster a favorable landscape for ongoing growth within the Infectious Disease Diagnostics Market, aligning with the shifting demands of healthcare systems globally.
Infectious Disease Diagnostics Market Restraining Factors:
Significant barriers in the Infectious Disease Diagnostics Market involve the elevated expenses associated with cutting-edge diagnostic technologies and the regulatory challenges that prolong the approval process for products.
The Infectious Disease Diagnostics sector encounters numerous challenges that could impede its development. One significant issue is the substantial expense associated with cutting-edge diagnostic technologies and procedures, which can restrict accessibility, especially in low- and middle-income nations. Moreover, the market is affected by regulatory obstacles and protracted approval processes for new diagnostic tools, delaying product introductions and creating unpredictability for manufacturers.
The existence of alternative diagnostic approaches, such as traditional clinical evaluations or more affordable rapid tests, also intensifies competition. Additionally, fluctuating rates of infectious disease prevalence across different regions contribute to an uneven demand for particular diagnostic solutions. Concerns regarding data privacy and the implementation of stringent data protection laws may further hinder technological progress, as companies are required to navigate complicated compliance requirements.
Furthermore, the swift mutation of pathogens raises concerns about the precision and dependability of currently available diagnostic tests, necessitating ongoing innovation and refinement. In spite of these hurdles, there is a growing emphasis on preventive healthcare, coupled with advancements in technologies like point-of-care testing and molecular diagnostics, which bode well for the market's future. Additionally, the current global focus on pandemic readiness and the monitoring of infectious diseases is likely to stimulate investment and encourage innovation, ultimately enhancing diagnostic capabilities.
Key Segments of the Infectious Disease Diagnostics Market
By Product
• Instruments
• Reagents, Kits & Consumables
• Services
By Technology
• Clinical Microbiology
• Polymerase Chain Reaction
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology
• Immunodiagnostics
• Others
By Application
• COVID-19
• Gastrointestinal Tract Infections
• Respiratory Diseases
• Sexually Transmitted Infection
• Tuberculosis
• Hospital-acquired Infection
• Mosquito-borne Diseases
• Others
By End-use
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Others
Regional Overview
North America
• US
• Canada
• Mexico
Europe
• Germany
• France
• U.K
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Rest of Asia Pacific
Middle East and Africa
• Saudi Arabia
• UAE
• Rest of Middle East and Africa
Latin America
• Brazil
• Argentina
• Rest of Latin America

