The global Lateral Flow Assay market is projected to reach $13.85 billion by 2027, growing at a CAGR of 8.8%
.jpg)
The global lateral flow assay market was valued at USD 6,025.8 million in 2019. Increase in prevalence of infectious diseases, growing geriatric population coupled with surging demand for rapid point-of-care diagnostics tests are key factors driving the global lateral flow assay market.
Lateral flow tests are widely used in human health for point-of-care testing. They can be performed by a healthcare professional or by the patient, and used in a range of settings including the laboratory informatics, clinic or home care setting. Various types of lateral flow assay are available in the market including kits & reagents, and lateral flow readers. Increase in infectious diseases such as malaria, rapid influenza diagnostics, HIV/AIDS and rising demand for home-based lateral flow assay are major factors propelling the growth of the market. Furthermore, rise in geriatric population fuels the need for early diagnosis and treatment of chronic diseases. This has led to increased demand for lateral flow assays. Moreover, lateral flow immunoassays offers benefits such as these are simple to use, flexible, allows rapid testing with multiplexing capabilities, and are cost-effective which is expected to propel market expansion in the future.
The global lateral flow assay market has been segmented based on product, application, technique, end-users, and region. Based on product, the global lateral flow assay market is categorized into kits & reagents, and lateral flow readers. In 2019, kits & reagents segment held dominant share of the global market. By application, clinical testing segment grabbed majority of the share of the global lateral flow assay market in 2019. Geographically, North America dominated the global lateral flow assay market in 2019, however, Asia Pacific is expected to be the fastest growing region by 2028.
Key players operating in the global lateral flow assay market include Siemens AG, Abbott Laboratories, Thermo Fischer Scientific, F.Hoffmann-La Roche, Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., bioMerieux, PerkinElmer, Qiagen N.V., Danaher Corporation, Hologic, Inc., and Quidel Corporation among others.
Lateral Flow Assay Market Scope
| Metrics | Details |
| Base Year | 2022 |
| Historic Data | 2017-2018 |
| Forecast Period | 2022-2027 |
| Study Period | 2017-2027 |
| Forecast Unit | Value (USD) |
| Revenue forecast in 2027 | $13.85 billion |
| Growth Rate | CAGR of 8.8% during 2017-2027 |
| Segment Covered | by Product, Regions |
| Regions Covered | North America, Europe, Asia Pacific, South America, Middle East and Africa |
| Key Players Profiled | Abbott Laboratories (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche AG (Switzerland), Siemens Healthineers AG (Germany), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), QIAGEN N.V. (Germany), PerkinElmer Inc. (U.S.), Quidel Corporation (U.S.) and Merck KGAA (Germany). |
Key Segment Of The Lateral Flow Assay Market
by Product, (USD Billion)
o Kits & Reagents
o Lateral Flow Readers
• Digital/Mobile Readers
• Benchtop Readers
by Technique, (USD Billion)
• Sandwich Assays
• Competitive Assays
• Multiplex Detection Assays
by Application, (USD Billion)
o Clinical Testing
• Infectious Disease Testing
• Cardiac Marker Testing
• Pregnancy & Fertility Testing
• Cholesterol Testing/Lipid Profile
• Drug Abuse Testing
• Other Clinical Tests
o Veterinary Diagnostics
o Food Safety & Environment Testing
o Drug Development & Quality Testing
Regional Overview, (USD Billion)
North America
• US
• Canada
Europe
• Germany
• France
• UK
• Rest of Europe
Asia Pacific
• China
• India
• Japan
• Rest of Asia Pacific
South America
• Mexico
• Brazil
• Rest of South America
Middle East and South Africa
Frequently Asked Questions (FAQ) :
The healthcare industry is witnessing a gradual shift in preference from traditional diagnosis to point-of-care diagnostics. The development of point-of-care (POC) devices that are portable, robust, easy to operate, battery powered, and able to monitor and detect infectious diseases is crucial. This will lead to minimize the diagnosis turnaround time and improve patient outcomes. The migration to using rapid diagnostics, in general, is on the increase with the estimated 93,000 healthcare professionals in the U.S leading the uptake in utilizing rapid diagnostic methods. For instance, Abingdon Health offers PCRD nucleic acid lateral flow immunoassays (NALFIA) for point of care testing in infectious disease applications, such as COVID-19. PCRD NALFIA assays detect specific labelled amplicons following an amplification sequence and provides high sensitivity with simple qualitative results. These assays are used in wide application areas such as infectious diseases, animal health, food and beverage, and environmental testing. Thus, development of advanced diagnostic solutions, such as immune & molecular diagnostics, and nanotechnology, which are quick & efficient will offer immense growth opportunities in the future.
The global lateral flow assay market has been segmented based on product, application, technique, end-users, and region. Based on product, the global lateral flow assay market is categorized into kits & reagents and lateral flow readers. Lateral flow readers segment is further sub-categorized into digital/mobile readers, and benchtop readers. Lateral flow readers have gained increased interest in diagnostic applications due to their various advantages in meeting the guidelines of the World Health Organization (WHO) for diagnostic tests in developing countries: inexpensive, sensitive, precise, user-friendly, rapid, durable, free of equipment and deliverable. Thus, a rise in the use of lateral flow readers to interpret precise results is expected to fuel market growth.
Application-wise, the global lateral flow assay is segmented into clinical testing, veterinary diagnostics, drug development & quality testing, food safety & environmental testing. In 2019, clinical testing segment grabbed majority of the share of the global market owing to rise in incidence of infectious diseases across the globe. On the basis of technique, the global lateral flow assay market is segregated into sandwich assays, competitive assays, and multiplex detection assays. Multiple detection assays segment is anticipated to grow at a rapid pace owing to detect multiple targets in a single test rather than using many individual tests.
On the basis of end-users, the global lateral flow assay market is segmented into hospitals, diagnostic centers, pharmaceutical & biotechnology companies, and home care. Hospitals held majority of the market share in 2019. Hospitals are well-equipped with technologically advanced equipment and devices to improve patient care and have availability of specialists. Moreover, increasing adoption of point-of-care testing in these facilities for rapid diagnosis and treatment will boost segment growth over the forecast period.
The global lateral flow assay market is cumulative to North America, Europe, Asia Pacific, Latin America and Middle East & Africa. North America dominated the global lateral flow assay market in 2019, however Asia Pacific is expected to grow at a highest pace through 2028.
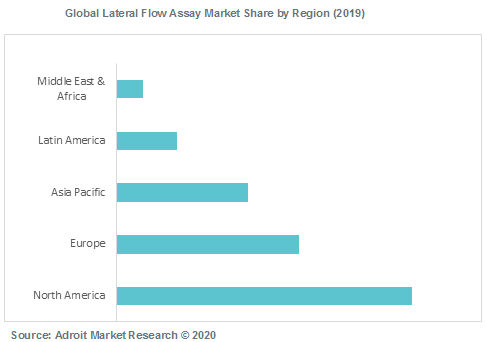
Rapid adoption of point-of-care tests, increase in incidences of infectious diseases, large geriatric population pool in the U.S. and Canada are factors anticipated to drive the lateral flow assay market in the region. According to Center for Disease Control and Prevention (CDC), 22.7 million individuals visited healthcare physicians for the diagnosis of influenza in the U.S. in 2017. Furthermore, presence of well-established industry players offering lateral flow immunoassays is other major factors likely to boost the growth of the market in North America.
However, Asia Pacific is expected to grow at a rapid pace over the next few years. There is a strong growth potential in the emerging economies such as India, and China. Increased cases of infectious diseases such as HIV/AIDS, Hepatitis, malaria and tuberculosis in low- and middle-income countries is expected to create huge growth opportunities for market players to tap these regions which will eventually foster the demand for lateral flow assay in the future.

