Market Analysis and Insights:
The market for Global Post Marketing Pharmocovigilence was estimated to be worth USD 5.58 billion in 2023, and from 2023 to 2032, it is anticipated to grow at a CAGR of 9.46%, with an expected value of USD 13.1 billion in 2032.
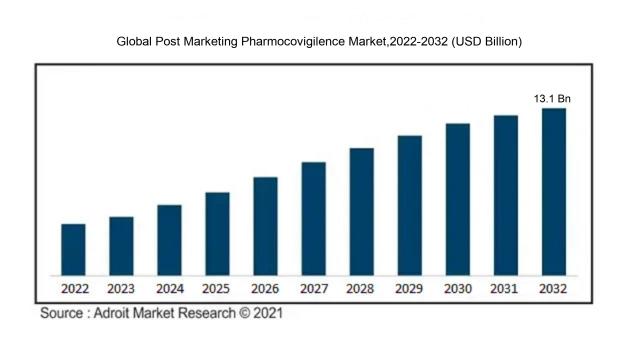
The post-marketing pharmacovigilance sector is being propelled by multiple factors. Firstly, the escalating introduction of new drugs into the market necessitates the critical monitoring of their safety and effectiveness. This is mainly due to the potential adverse reactions and side effects that these medications may have on patients. Secondly, the increasing awareness among both the general public and healthcare professionals regarding the significance of monitoring drug safety has contributed to the rising demand for post-marketing pharmacovigilance services. Furthermore, stringent regulations imposed by various government bodies and organizations to safeguard patient well-being have accelerated market expansion. The surge in the global elderly population and the resulting uptick in chronic disease incidence have boosted medication consumption, consequently increasing the need for post-marketing pharmacovigilance services. Lastly, the incorporation of advanced technologies such as artificial intelligence and big data analytics into pharmacovigilance procedures has notably enhanced the efficiency and efficacy of drug safety monitoring, further driving market growth.
Post Marketing Pharmocovigilence Market Scope :
| Metrics | Details |
| Base Year | 2023 |
| Historic Data | 2018-2022 |
| Forecast Period | 2024-2032 |
| Study Period | 2018-2032 |
| Forecast Unit | Value (USD) |
| Revenue forecast in 2032 | USD 13.1 billion |
| Growth Rate | CAGR of 9.46% during 2023-2032 |
| Segment Covered | By Type, By Product, By End-use, By Region. |
| Regions Covered | North America, Europe, Asia Pacific, South America, Middle East and Africa |
| Key Players Profiled | QuintilesIMS, Accenture, LabCorp (Covance), Cognizant, IBM Corporation, IQVIA, Boehringer Ingelheim International GmbH, ICON Plc, PAREXEL International Corporation, PRA Health Sciences, and United BioSource Corporation |
Market Definition
Pharmacovigilance in the post-marketing phase encompasses the ongoing surveillance and appraisal of a drug's safety and efficacy following its approval and market release. It entails the identification, evaluation, and mitigation of potential hazards related to drug consumption through the examination of empirical data, as well as the advocacy for suitable precautions to ensure the safe utilization of pharmaceuticals.
Post-approval pharmacovigilance, also referred to as drug safety surveillance, is a pivotal component of the healthcare landscape. This method encompasses the organized gathering, analysis, and appraisal of data surrounding the safety and efficacy of pharmaceutical treatments subsequent to their endorsement and availability to the populace. It carries immense significance in recognizing and managing any plausible adverse reactions or hazards linked with these medications, guaranteeing patient welfare and advancing public health. Through the consistent scrutiny and interpretation of information on drug administration and its consequences on individuals, post-approval pharmacovigilance enables the identification and mitigation of potential risks or side effects that might not have been evident during initial clinical trials. This continuous monitoring plays a key role in updating concerned ies, including regulatory bodies, healthcare professionals, and patients, about essential revisions to safety protocols, labeling, and directives.
Furthermore, it aids in the identification of potential medication-related challenges such as drug interferences, resistance development, or necessity for usage restrictions, facilitating prompt implementation of remedial and control measures. In essence, post-approval pharmacovigilance stands as a critical element in ensuring the sustained, safe usage of pharmaceuticals and enhancing patient well-being.
Key Market Segmentation:

Insights On Key Type
Spontaneous Reporting
Spontaneous Reporting is expected to dominate the Global Post Marketing Pharmacovigilance Market. Spontaneous Reporting is a passive surveillance system that relies on healthcare professionals and consumers to voluntarily report adverse drug reactions (ADRs) to regulatory authorities. It is the most widely used and established method for collecting safety data in pharmacovigilance. This system allows for the identification of previously unknown or rare ADRs, providing important insights into the safety profile of drugs. Given its long-standing usage and widespread acceptance, Spontaneous Reporting is likely to continue as the dominant in the Global Post Marketing Pharmacovigilance Market.
Intensified ADR Reporting
Intensified ADR Reporting, also known as targeted or enhanced reporting, is a within the By Type category of the Global Post Marketing Pharmacovigilance Market. This approach involves active surveillance and more frequent reporting of suspected adverse events, typically in specific patient populations or drug classes. While Intensified ADR Reporting offers valuable insights into safety concerns in targeted populations, it is not expected to dominate the Global Post Marketing Pharmacovigilance Market. Its focus on specific groups limits its widespread applicability and reach compared to Spontaneous Reporting.
Targeted Spontaneous Reporting
Targeted Spontaneous Reporting is one more noticeable within the By Type category of the Global Post Marketing Pharmacovigilance Market. It combines the principles of Spontaneous Reporting with targeted data collection efforts. Targeted Spontaneous Reporting is conducted in specific patient populations, geographic regions, or for specific drug classes, with the aim of capturing safety signals more effectively. While this approach enhances the quality of the data collected, it is not anticipated to dominate the Global Post Marketing Pharmacovigilance Market due to its narrower focus compared to Spontaneous Reporting.
Cohort Event Monitoring
Cohort Event Monitoring is a within the By Type category of the Global Post Marketing Pharmacovigilance Market. It involves the prospective monitoring of safety outcomes in defined patient populations over an extended period. This approach typically uses electronic health records and other healthcare databases to identify and monitor potential adverse events associated with medications. However, Cohort Event Monitoring is not expected to dominate the Global Post Marketing Pharmacovigilance Market due to its more limited scope and applicability compared to Spontaneous Reporting.
EHR Mining
EHR Mining, or Electronic Health Record Mining, is a within the By Type category of the Global Post Marketing Pharmacovigilance Market. It involves the systematic analysis of electronic health records to identify potential safety signals associated with medications. While EHR Mining has the potential to contribute valuable safety information, it is unlikely to dominate the Global Post Marketing Pharmacovigilance Market. Its reliance on available electronic health records and the challenges associated with data privacy and standardization limit its widespread adoption and impact compared to Spontaneous Reporting.
Insights On Key Product
Online Media
Online media is expected to dominate the global post marketing pharmacovigilance market. With the advancement of technology and the widespread use of the internet, online platforms have become a primary source for accessing information. The ease of accessing online media, such as websites, blogs, social media platforms, and online journals, allows for quick and efficient dissemination of information related to pharmacovigilance. Moreover, online media has the potential to reach a wider audience and facilitate real-time communication, enabling healthcare professionals, researchers, and pharmaceutical companies to stay updated on the latest drug safety information. Therefore, online media is expected to be the dominating in the global post marketing pharmacovigilance market.
Books
Although online media is expected to dominate the global post marketing pharmacovigilance market, books continue to hold significance in this field. Books provide in-depth knowledge and comprehensive information on pharmacovigilance practices, regulations, and procedures. They serve as a valuable resource for healthcare professionals, researchers, and students who seek a more detailed understanding of drug safety and surveillance. While online media provides quick access to updated information, books offer a comprehensive and timeless reference that may be preferred by some individuals. Despite their enduring value, books may not dominate the market as much as online media, considering the rapidly evolving nature of pharmacovigilance practices.
Journals
Journals also play a crucial role in the post marketing pharmacovigilance field. They serve as a platform for publishing research studies, clinical trials, and case reports related to drug safety and adverse reactions. Journals provide a credible source of information and allow for the peer-reviewed dissemination of new findings in pharmacovigilance. Researchers and healthcare professionals often rely on journals to access evidence-based information and stay updated on the latest developments in the field. However, journals may not dominate the global post marketing pharmacovigilance market as much as online media since their accessibility and dissemination speed may be relatively slower compared to online platforms.
Insights On Key End-use
Hospitals
Hospitals is expected to dominate the Global Post Marketing Pharmacovigilance Market. Hospitals play a crucial role in pharmacovigilance as they are responsible for the direct treatment of patients and administration of drugs. With access to a large patient pool, hospitals are likely to have a higher volume of drug usage and adverse events reporting. Additionally, hospitals have well-established systems for monitoring and reporting adverse drug reactions, making them an essential component of the post-marketing pharmacovigilance process.
Research Organizations
Research organizations, although important in the field of pharmacovigilance, are not expected to dominate the Global Post Marketing Pharmacovigilance Market. Research organizations primarily focus on conducting clinical trials and generating data on the safety and effectiveness of new drugs before they are released into the market. While they contribute valuable insights and expertise, their involvement in post-marketing pharmacovigilance is often limited to specific research studies rather than overall market dominance.
Insights on Regional Analysis:
Europe
Europe is expected to dominate the Global Post Marketing Pharmacovigilance market. This can be attributed to several factors. Firstly, Europe has a large pharmaceutical industry with a robust regulatory framework in place, which emphasizes the importance of post-marketing surveillance and safety monitoring. The European Medicines Agency (EMA) plays a crucial role in ensuring drug safety and monitoring adverse events. Additionally, Europe has a high level of awareness and understanding of the importance of pharmacovigilance among healthcare professionals and patients. The region also has well-established pharmacovigilance databases, such as the EudraVigilance system, which enables the collection, analysis, and reporting of adverse drug reactions. Furthermore, Europe has a strong focus on research and development in healthcare, leading to the introduction of innovative drugs, thereby driving the demand for post-marketing pharmacovigilance services.
North America
North America is another significant region in the Global Post Marketing Pharmacovigilance market. The region is characterized by a well-developed healthcare infrastructure and a rigorous regulatory framework. The Food and Drug Administration (FDA) in the United States plays a pivotal role in ensuring drug safety and monitoring adverse events. Moreover, the growing prevalence of chronic diseases and the rising demand for novel therapies in North America contribute to the increased need for comprehensive pharmacovigilance services. Additionally, advancements in technology and digital healthcare systems in the region support efficient post-marketing surveillance and signal detection. The adoption of electronic health records and data mining techniques enables the identification and analysis of adverse drug reactions effectively.
Asia Pacific
Asia Pacific is a region that holds immense potential in the Global Post Marketing Pharmacovigilance market. With a large population and a growing pharmaceutical industry, the region presents numerous opportunities for post-marketing surveillance and safety monitoring. The increasing adoption of pharmaceutical products, coupled with a rising awareness of adverse drug reactions, drives the demand for pharmacovigilance services in Asia Pacific. Furthermore, regulatory agencies in countries like Japan, India, and China are stepping up efforts to strengthen pharmacovigilance systems and ensure patient safety. The presence of contract research organizations and outsourcing services in the region also supports the growth of the pharmacovigilance market.
Latin America
Latin America is emerging as a significant player in the Global Post Marketing Pharmacovigilance market. The region is witnessing a growing emphasis on drug safety regulations, with regulatory agencies like the Brazilian Health Regulatory Agency (ANVISA) and the National Institute for Surveillance of Medications and Health Products (INVIMA) in Colombia strengthening their pharmacovigilance systems. Additionally, the increasing availability and accessibility of healthcare services in Latin American countries contribute to the demand for pharmacovigilance activities. The region's large population and rising disease burden further boost the need for post-marketing surveillance and safety monitoring services.
Middle East & Africa
The Middle East & Africa region is gradually recognizing the importance of post-marketing pharmacovigilance. Although the market is still developing, efforts are being made to establish and enhance pharmacovigilance systems in countries like Saudi Arabia, South Africa, and the United Arab Emirates. The growing pharmaceutical industry and the presence of multinational companies investing in the region contribute to the demand for pharmacovigilance services. Furthermore, collaborations between local regulatory authorities and global organizations facilitate knowledge sharing and capacity building in pharmacovigilance. As healthcare systems continue to evolve in the Middle East & Africa, the demand for effective post-marketing surveillance and safety monitoring is expected to grow.
Company Profiles:
The significant contributors in the worldwide Post Marketing Pharmacovigilance sector have a pivotal role in overseeing and assessing the safety and effectiveness of medicinal products once they have received approval for market distribution. Their duties encompass the gathering and examination of information on negative drug reactions to uphold patient well-being and adhere to regulatory standards.
Prominent stakeholders in the Post-Marketing Pharmacovigilance sector encompass QuintilesIMS, Accenture, LabCorp (Covance), Cognizant, IBM Corporation, IQVIA, Boehringer Ingelheim International GmbH, ICON Plc, PAREXEL International Corporation, PRA Health Sciences, and United BioSource Corporation. These organizations actively engage in post-marketing pharmacovigilance activities, rendering services such as drug safety consultancy, case processing, signal identification, risk management, compliance with regulations, and reporting of adverse events. Their specialized assistance is directed towards pharmaceutical and biotechnology firms to aid in the supervision and regulation of safety profiles associated with marketed medications. Through their extensive industry knowledge and technological advancements, these pivotal entities hold a significant role in upholding public health by ensuring the safety and effectiveness of pharmaceutical goods during the post-marketing phase.
COVID-19 Impact and Market Status:
The global emphasis on post-marketing pharmacovigilance has ened significantly due to the Covid-19 pandemic, prompting a greater need for monitoring the safety and effectiveness of pharmaceutical goods on a worldwide scale.
The global economy has experienced profound effects from the COVID-19 pandemic, with the pharmaceutical industry being icularly impacted. Post-marketing pharmacovigilance, tasked with overseeing the safety and efficacy of medications post-approval, has undergone notable shifts. The ened emphasis on creating and disseminating COVID-19 vaccines and treatments has redirected resources away from other pharmacovigilance areas, causing delays in adverse drug reaction data collection and analysis. Healthcare system disruptions and decreased patient interactions with physicians during the pandemic have further complicated the reporting of medication-related adverse events. Additionally, the expedited development and emergency approval of new drugs and vaccines have raised concerns about their long-term safety assessment. Overall, the pandemic has strained post-marketing pharmacovigilance, challenging its ability to effectively monitor and safeguard medication usage.
Latest Trends and Innovation:
- In October 2021, Oracle Corporation announced the acquisition of Palerra, a leading provider of cloud-based security solutions for enterprise organizations.
- In August 2021, IQVIA Holdings Inc. acquired Linguamatics, a healthcare AI company that provides natural language processing (NLP) text mining.
- In March 2021, Accenture acquired CynergisTek, a recognized leader in healthcare cybersecurity consulting services.
- In February 2021, ProPharma Group acquired Craigavon-based Ardena, a specialist contract development and manufacturing organization.
- In January 2021, Pfizer Inc. announced the acquisition of Amplyx Pharmaceuticals, a biotech company focused on developing therapies for patients with compromised immune systems.
- In December 2020, Syneos Health acquired Synteract, a contract research organization specializing in clinical development solutions for emerging biopharma companies.
- In November 2020, LabCorp completed the acquisition of Myriad RBM, a leading multiplex immunoassay testing laboratory.
- In September 2020, Siemens Healthineers AG acquired Varian Medical Systems, a global provider of radiation oncology treatments and software, to strengthen its cancer care portfolio.
- In June 2020, MedPro Systems, a subsidiary of Pharmaceutical Strategies Group (PSG), acquired MD Mindset, a provider of digital-based patient engagement solutions for healthcare professionals.
- In January 2020, Parexel International completed its acquisition of Synchrogenix, a leading provider of regulatory writing services.
Significant Growth Factors:
The expansion drivers of the Post-Marketing Pharmacovigilance Market can be credited to the growing focus on medication safety, the increasing incidence of negative drug responses, and progressions in technology.
The Post Marketing Pharmacovigilance (PV) Market is poised for substantial expansion in the foreseeable future due to various factors. Primarily, the pharmaceutical industry's ened prioritization of drug safety and adherence to regulatory standards is propelling the need for robust PV mechanisms. The surge in adverse drug reactions (ADRs) and the imperative for precise reporting and analysis of such incidents are prompting companies to invest in sophisticated PV technologies and solutions.
Additionally, the escalating variety of drugs available on the market and the intricacy of drug regimens have intensified the necessity for efficient PV frameworks. With the continuous influx of drugs into the market and the resultant increased probability of unforeseen adverse occurrences, there is a growing requirement for pharmacovigilance services to identify, evaluate, and mitigate these risks to safeguard patient well-being.
Moreover, the global enforcement of stringent regulatory frameworks has fostered a favorable climate for the expansion of the PV sector. Regulatory bodies like the FDA, EMA, and MHRA have established protocols mandating the surveillance and notification of drug safety data. Consequently, pharmaceutical entities are increasingly embracing PV systems to adhere to these regulations, mitigating the possibility of penalties or product recalls.
Furthermore, technological advancements, including artificial intelligence (AI), big data analytics, and cloud computing, have brought about a transformation in the PV landscape. These innovations facilitate swifter and more accurate identification of adverse incidents, signal detection, and data analysis, thereby enhancing the efficiency and efficacy of PV processes.
Restraining Factors:
Insufficient knowledge among healthcare practitioners and the absence of strict regulations present notable obstacles to the advancement of the Post
Marketing Pharmacovigilance Market.
The growth of the Post-Marketing Pharmacovigilance Market is encountering impediments despite its upward trajectory. An inherent challenge lies in the insufficient knowledge among medical practitioners and patients regarding the significance of pharmacovigilance, resulting in underreporting of adverse drug reactions (ADRs) and impeding the efficacy of surveillance post-market. The intricate regulatory landscape and heterogeneous pharmacovigilance guidelines across different geographical areas also impede global pharmacovigilance initiatives. Scarce resources allocated to pharmacovigilance activities, encompassing workforce, technology, and infrastructure, hinder the seamless collection, analysis, and detection of signals, thereby diminishing the overall effectiveness of post-market surveillance systems. Furthermore, the underutilization of advanced technologies like artificial intelligence and machine learning in pharmacovigilance processes delays the prompt identification and examination of ADRs. Lastly, apprehensions pertaining to patient data privacy and confidentiality in the context of pharmacovigilance data collection and utilization can deter active engagement and impede data exchange. Despite these challenges, the Post-Marketing Pharmacovigilance Market holds substantial growth potential. Elevating awareness levels, streamlining regulations, augmenting investments in resources and technology, and establishing robust data protection and privacy protocols can surmount these hurdles and steer the market towards a promising future.
Key Segments of the Post Marketing Pharmocovigilence Market
Type Overview
• Spontaneous Reporting
• Intensified ADR Reporting
• Targeted Spontaneous Reporting
• Cohort Event Monitoring
• EHR Mining
Product Overview
• Books
• Online Media
• Journals
End-Use Overview
• Hospitals
• Research Organizations
Regional Overview
North America
• US
• Canada
• Mexico
Europe
• Germany
• France
• U.K
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Rest of Asia Pacific
Middle East and Africa
• Saudi Arabia
• UAE
• Rest of Middle East and Africa
Latin America
• Brazil
• Argentina
• Rest of Latin America

