Market Analysis and Insights:
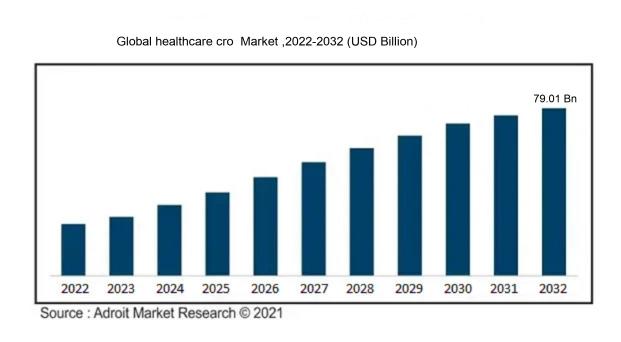
The market for global healthcare CRO was estimated to be worth USD 44.01 billion in 2022, and from 2022 to 2032, it is anticipated to grow at a CAGR of 6.01%, with an expected value of USD 79.01 billion in 2032.
The healthcare Contract Research Organization (CRO) sector derives its growth from a variety of factors. The primary driver is the escalating need for novel and efficacious treatments, medications, and therapies, which has ened the requirement for clinical research and development.
Pharmaceutical and biotechnology firms are striving to introduce new products to the market, necessitating the specialized services and knowledge offered by CROs. Additionally, the trend of pharmaceutical companies outsourcing clinical trial operations has bolstered market expansion, enabling cost reduction and access to the specialized capabilities and resources available through CRO nerships. Moreover, the increasingly stringent regulatory landscape has amplified the necessity for companies to engage CROs in navigating intricate compliance protocols. The evolution of technology, including electronic data capture systems, has enhanced the efficiency and precision of clinical trials, thereby spurring the demand for CRO services. Furthermore, the rising incidence of chronic illnesses and the aging global population have intensified the urgency for clinical trials and drug development, thereby propelling the healthcare CRO market. In conclusion, the growth of the healthcare CRO market is predominantly steered by the pursuit of innovative therapies, the trend of outsourcing, adherence to regulatory standards, technological progress, and shifting demographic patterns.
healthcare cro Market scope:
| Metrics | Details |
| Base Year | 2023 |
| Historic Data | 2018-2022 |
| Forecast Period | 2024-2032 |
| Study Period | 2018-2032 |
| Forecast Unit | Value (USD) |
| Revenue forecast in 2032 | USD 79.01 billion |
| Growth Rate | CAGR of 6.01% during 2022-2032 |
| Segment Covered | By Type, By Service, By Therapeutic Area, By End-User, By Region. |
| Regions Covered | North America, Europe, Asia Pacific, South America, Middle East and Africa |
| Key Players Profiled | IQVIA Holdings Inc., Laboratory Corporation of America Holdings, Syneos Health Inc., Medpace Holdings Inc., PAREXEL International Corporation, ICON PLC, PPD Inc., Charles River Laboratories International Inc., WuXi AppTec Inc., and Siro Clinpharm Pvt. Ltd. |
Market Definition
A healthcare Contract Research Organization (CRO) offers essential support to pharmaceutical, biotechnology, and medical device sectors by managing clinical trials and research operations on their behalf. These organizations are instrumental in facilitating the development, testing, and regulatory adherence of innovative healthcare products for market introduction.
The position of Healthcare Chief Revenue Officer (CRO) is a critical one within the healthcare sector, serving as a key strategic leader responsible for overseeing the financial operations of healthcare entities. In this role, the CRO is tasked with various duties including revenue enhancement, financial strategy development, and process enhancement, all aimed at ensuring the financial health and advancement of healthcare organizations. Through the optimization of revenue cycles, data analysis, and identification of revenue sources, the CRO contributes to enhancing operational effectiveness, advancing patient care, and maximizing financial outcomes. Additionally, the CRO collaborates closely with executive teams to create and implement revenue-generating initiatives while upholding compliance with regulatory standards. Ultimately, the healthcare CRO's role is indispensable in fostering financial sustainability, operational efficacy, and the delivery of high-quality healthcare services to patients.
Key Market Segmentation:

Insights On Key Type
Pre-Clinical
Among the s of the By Type category, Pre-Clinical is expected to dominate the global healthcare CRO market. The Pre-Clinical encompasses the early stages of drug development, including laboratory-based research and animal testing. This plays a crucial role in determining the safety and effectiveness of potential drugs before they progress to human clinical trials. The demand for Pre-Clinical services is driven by the increasing need for drug discovery and development, as well as regulatory requirements for assessing the toxicity and efficacy of new compounds. Additionally, advancements in research technologies and the growing investment in pharmaceutical R&D further contribute to the dominance of the Pre-Clinical .
Drug Discovery
In the healthcare CRO market, the Drug Discovery holds significant importance as it involves the identification and development of novel drug candidates. However, it is not expected to dominate the global healthcare CRO market. The Drug Discovery focuses on early-stage research activities, such as target identification, lead optimization, and medicinal chemistry. While drug discovery is a crucial step in the development of new therapies, its dominance is limited by the substantial resources and expertise required to bring a drug from discovery to commercialization. The Drug Discovery often collaborates with other s, icularly Pre-Clinical and Clinical, to progress the drug development pipeline.
Clinical
The Clinical plays a vital role in evaluating the safety and efficacy of drugs in human subjects through rigorous testing in clinical trials. However, it is not expected to dominate the global healthcare CRO market. Clinical trials are conducted in multiple phases to gather data on drug safety, dosage, and effectiveness. The Clinical works closely with other s, such as Pre-Clinical and Drug Discovery, to ensure the successful transition from pre-clinical research to human trials. The demand for Clinical services is driven by the increasing complexity of regulatory compliance, globalization of clinical trials, and rising patient-centric approaches. While Clinical is an integral of the drug development process, its dominance is limited due to the significant investments and timelines associated with clinical trials.
Insights On Key Service
Project Management/Clinical Supply Management: This is expected to dominate the Global healthcare CRO market. The increasing complexity of clinical trials and the need for effective management and coordination have propelled the demand for project management and clinical supply management services. These services involve developing project plans, allocating resources, ensuring timely delivery of supplies, and managing logistics. With the growing number of clinical trials and the need for efficient project execution, the demand for project management and clinical supply management services is expected to remain high in the healthcare CRO market.
Data Management: Data management is another significant in the healthcare CRO market. It involves the collection, storage, and analysis of clinical trial data. With the emphasis on data integrity, accuracy, and compliance with regulatory guidelines, there is a growing need for robust data management solutions. The availability of advanced technologies and software solutions enables efficient data management, leading to increased demand for these services in the healthcare CRO market.
Regulatory/Medical Affairs: Regulatory and medical affairs services play a critical role in the healthcare CRO market. These services involve ensuring compliance with regulatory requirements, managing submissions and approvals, and providing medical writing support. With the increasing focus on safety and efficacy of drugs, pharmaceutical companies are relying heavily on regulatory and medical affairs services offered by CROs. The changing regulatory landscape and the need for expert guidance drive the demand for regulatory/medical affairs services in the healthcare CRO market.
Medical Writing: Medical writing is a specialized in the healthcare CRO market. It involves the development of various documents, including clinical study protocols, regulatory submissions, and scientific manuscripts. The demand for medical writing services is driven by the need for accurate, concise, and compliant documentation throughout the drug development process. Pharmaceutical companies rely on CROs for their medical writing expertise, contributing to the dominance of this in the healthcare CRO market.
Clinical Monitoring: Clinical monitoring is an essential in the healthcare CRO market. It involves overseeing and managing clinical trial activities to ensure icipant safety, data accuracy, and protocol compliance. The increasing complexity of clinical trials and the need for reliable data have led to a surge in demand for clinical monitoring services. CROs with experienced clinical monitoring professionals are well-positioned to dominate this in the healthcare CRO market.
Quality Management/Assurance: Quality management/assurance is a crucial in the healthcare CRO market. It involves implementing quality systems, conducting audits, and ensuring adherence to Good Clinical Practice (GCP) guidelines. With the regulatory scrutiny on clinical trial conduct and data integrity, pharmaceutical companies rely on CROs to provide comprehensive quality management/assurance services. The ability to deliver high-quality services and ensure compliance positions CROs favorably in this of the healthcare CRO market.
Bio-statistics: Bio-statistics is an important in the healthcare CRO market. It involves the application of statistical methods to analyze and interpret clinical trial data. The growing emphasis on data-driven decision-making and evidence-based medicine has led to an increased demand for bio-statistical expertise. CROs offering robust bio-statistical services are expected to be dominant players in this of the healthcare CRO market.
Investigator Payments: Investigator payments are a significant aspect of the healthcare CRO market. They involve managing and disbursing payments to clinical trial investigators for their icipation. As the number of clinical trials and investigators involved in research activities continue to increase, there is a corresponding need for efficient investigator payment services. CROs specializing in investigator payment management are expected to play a dominant role in this of the healthcare CRO market.
Laboratory: Laboratory services in the healthcare CRO market encompass a wide range of activities, including sample analysis, biomarker testing, and genetic research. CROs offering comprehensive laboratory services cater to the growing demand for specialized testing and analysis in clinical trials. The ability to provide accurate and reliable laboratory services contributes to the dominance of CROs in this of the healthcare CRO market.
Patient and Site Recruitment: Patient and site recruitment services play a critical role in the success of clinical trials. CROs specializing in patient and site recruitment leverage their networks, outreach strategies, and patient databases to identify and recruit suitable participants for clinical trials. As the recruitment of eligible participants remains a significant challenge in clinical research, CROs offering efficient patient and site recruitment services are expected to dominate this in the healthcare CRO market.
Technology: Technology services encompass a range of digital solutions, software platforms, and IT infrastructure required for efficient clinical trial management. CROs offering advanced technology solutions that streamline processes, enable real-time data access, and improve collaboration are well-positioned to dominate this in the healthcare CRO market. The integration of technology-driven services and solutions is crucial for efficient clinical trial execution.
Others: The "Others" encompasses a variety of services that are less prominent in the healthcare CRO market, such as pharmacovigilance, clinical trial supply chain management, and patient engagement services. While these services play important roles in clinical research, their relative market share might be smaller compared to the dominating s mentioned earlier. The dominance of the healthcare CRO market is likely to be driven by the s mentioned above, while the "Others" might have a relatively smaller share.
Insights On Key Therapeutic Area
Oncology is expected to dominate the Global healthcare cro Market. With the increasing prevalence of cancer and the continuous development of innovative cancer treatments, the demand for CRO services in the oncology therapeutic area is projected to be the highest. Oncology clinical trials require specific expertise in terms of patient recruitment, protocol design, and data management, making it a complex and specialized field within the healthcare CRO market. Moreover, the high cost of cancer drugs and the need for efficient clinical trial management further contribute to the dominance of oncology in the global healthcare CRO market.
Cardiovascular:
Cardiovascular diseases are a significant healthcare burden worldwide. However, compared to oncology, the cardiovascular therapeutic area is not expected to dominate the global healthcare CRO market. While cardiovascular clinical trials are crucial for developing new treatment strategies and assessing the safety and efficacy of cardiovascular therapies, the demand for CRO services in this area may be overshadowed by the higher demand in oncology. Nevertheless, the cardiovascular therapeutic area still represents a significant within the healthcare CRO market, with ongoing clinical trials focusing on disease prevention, treatment optimization, and the development of innovative cardiovascular therapies.
Infectious Diseases:
The infectious diseases therapeutic area plays a vital role in public health, but it is not expected to dominate the global healthcare CRO market. Although infectious diseases, such as COVID-19, remain a global concern, the duration of clinical trials in this area is relatively shorter compared to other therapeutic areas like oncology. The urgency to develop treatments and vaccines for infectious diseases may lead to more in-house research efforts by pharmaceutical companies, impacting the demand for CRO services in this . However, the global healthcare CRO market continues to support clinical trials related to infectious diseases, including research on antiviral therapies, diagnostics, and vaccine development.
Neurology:
Neurology is an essential therapeutic area, encompassing a wide range of disorders affecting the central nervous system. However, it is not expected to dominate the global healthcare CRO market. Neurological clinical trials often involve complex study designs, intricate patient selection criteria, and specialized assessment measures, making them resource-intensive and time-consuming. Additionally, the relatively small patient populations and the challenges in recruiting suitable candidates for neurological trials can limit the demand for CRO services in this . Nevertheless, the importance of advancing neurology research and finding novel treatments for neurological disorders continues to drive clinical trial activities supported by the healthcare CRO market.
Insights On Key End-User
Pharmaceutical Industries
Among the s of By End-User, the Pharmaceutical Industries are expected to dominate the Global healthcare cro Market. These industries play a vital role in the development, production, and distribution of pharmaceutical drugs. With the increasing demand for new drugs and treatments, pharmaceutical companies are actively engaged in clinical research to ensure the safety and efficacy of their products. They invest major resources in healthcare cro services to conduct clinical trials, data management, and regulatory compliance. The pharmaceutical sector's dominance can be attributed to its substantial financial resources, extensive research and development capabilities, and strong collaborations with healthcare cro service providers.
Biotech Industries
In the Global healthcare cro Market, Biotech Industries represent a significant . Biotechnology companies focus on developing innovative technologies and products to improve healthcare outcomes. They utilize healthcare cro services to conduct preclinical and clinical trials, collect and analyze data, and ensure regulatory compliance. Biotech industries have been experiencing steady growth due to increased investments in research and development, advancements in biotechnology, and the need for personalized medicine. The collaboration between biotech companies and healthcare cro service providers drives the growth of this .
Medical Device Industries
The Medical Device Industries also form a crucial of the Global healthcare cro Market. These industries are responsible for designing, developing, and manufacturing medical devices used in healthcare settings. To ensure the safety and effectiveness of their devices, medical device companies rely on healthcare cro services for clinical trials, validation studies, and regulatory submissions. The demand for innovative medical devices, technological advancements, and stringent regulatory requirements contribute to the growth of this .
Academic Institutions
While Pharmaceutical Industries, Biotech Industries, and Medical Device Industries dominate the Global healthcare cro Market, Academic Institutions also play a significant role. Academic institutions are involved in research and development activities, including clinical trials, to advance medical knowledge, develop new treatments, and train future healthcare professionals. Although academic institutions may not have the same level of financial resources or commercial focus as the other s, their contributions to healthcare cro cannot be overlooked. By conducting research, generating data, and collaborating with other stakeholders, academic institutions contribute to the overall growth and advancements in the Global healthcare cro Market.
Insights on Regional Analysis:
North America is expected to dominate the global healthcare CRO market. This region has a well-developed healthcare industry, with advanced technology, strong research infrastructure, and a high demand for clinical trials. The presence of key market players, such as IQVIA, Laboratory Corporation of America Holdings, and ICON plc, further strengthens the position of North America in the market. Additionally, the favorable regulatory environment, robust healthcare expenditure, and increasing focus on precision medicine and personalized healthcare contribute to the dominance of North America in the global healthcare CRO market.
Asia Pacific:
The Asia Pacific region is witnessing significant growth in the healthcare CRO market. This can be attributed to the rising healthcare infrastructure development, increasing outsourcing activities, and growing demand for cost-effective clinical trials in countries like India and China. The region also benefits from a large and diverse patient population, which presents ample opportunities for clinical research studies. Furthermore, the availability of skilled professionals at relatively lower costs and the presence of regulatory reforms and support from government organizations are driving the growth of the healthcare CRO market in Asia Pacific.
Europe:
Europe has a mature healthcare sector and is expected to have a substantial share in the global healthcare CRO market. The region boasts a strong research and development infrastructure, well-established regulatory frameworks, and a high level of clinical expertise. The presence of prominent clinical research organizations and contract research organizations, along with collaborations between academia and industry, further promote the growth of the healthcare CRO market in Europe. Moreover, the increasing prevalence of chronic diseases, rising clinical research outsourcing, and government initiatives to accelerate drug development and approval processes contribute to the dominance of Europe in the global healthcare CRO market.
Latin America:
Latin America is witnessing steady growth in the healthcare CRO market. Factors such as a large and diverse patient population, improving healthcare infrastructure, and the presence of skilled professionals are contributing to the region's growth. Additionally, cost advantages, less stringent regulatory requirements, and favorable government policies are attracting pharmaceutical companies to conduct clinical trials in Latin America. Moreover, increasing awareness about clinical research and rising investments in biotechnology and pharmaceutical sectors further support the growth of the healthcare CRO market in the region.
Middle East & Africa:
The Middle East & Africa region is emerging as a potential market for healthcare CRO. The region's healthcare infrastructure and research capabilities have been expanding rapidly in recent years. Countries like South Africa, Saudi Arabia, and the United Arab Emirates are at the forefront of clinical research activities in the region. Factors such as rising healthcare expenditure, increasing prevalence of chronic diseases, and government initiatives to improve healthcare services are driving the growth of the healthcare CRO market in the Middle East & Africa. Moreover, collaborations with international research organizations and initiatives to promote clinical trials are further fueling the market growth in this region.
Company Profiles:
Contract research organizations (CROs) are vital contributors to the global healthcare sector, offering clinical trial services and research proficiency to pharmaceutical and biotechnology firms. These companies, central to the global healthcare CRO market, delegate clinical research initiatives to CROs for streamlined drug advancement and adherence to regulatory standards.
Leading firms in the healthcare Contract Research Organization (CRO) sector comprise IQVIA Holdings Inc., Laboratory Corporation of America Holdings, Syneos Health Inc., Medpace Holdings Inc., PAREXEL International Corporation, ICON PLC, PPD Inc., Charles River Laboratories International Inc., WuXi AppTec Inc., and Siro Clinpharm Pvt. Ltd. These organizations are actively engaged in delivering comprehensive clinical trial assistance, data management, regulatory conformity, and other research and development undertakings to the pharmaceutical and biotechnology sectors. Through their diverse range of competencies and extensive global presence, these prominent entities significantly contribute to expediting drug advancement and facilitating the introduction of life-saving therapies to the marketplace.
COVID-19 Impact and Market Status:
The global healthcare CRO market has experienced notable effects due to the Covid-19 pandemic, resulting in a rise in the need for clinical trials and data management services.
The healthcare Contract Research Organization (CRO) sector has been significantly impacted by the COVID-19 pandemic. There has been a notable upsurge in the demand for clinical trials and research services, driven by the urgent race among pharmaceutical and biotechnology firms to develop and evaluate potential treatments and vaccines for COVID-19. Consequently, there has been a ened need for CRO services, encompassing clinical trial oversight, data management, and regulatory assistance. Nevertheless, the industry has faced numerous challenges due to the pandemic. Issues such as travel restrictions, lockdowns, and social distancing measures have disrupted activities like site monitoring, patient recruitment, and data gathering.
Furthermore, the focus on COVID-19 research has led to the delay or cancellation of non-COVID-19 studies, impacting the revenue of CROs. Despite these obstacles, the healthcare CRO market is projected to bounce back and experience growth post-pandemic, as the outsourcing of clinical research services remains vital for effective drug development and regulatory adherence.
Latest Trends and Innovation:
- In March 2021, ICON plc acquired PRA Health Sciences, creating a leading provider of outsourced drug development services.
- In October 2020, IQVIA Holdings Inc. announced the acquisition of a majority stake in Research & Development Solutions (RDS) to enhance its capabilities in patient-centered research.
- In September 2020, LabCorp completed the acquisition of Envigo's non-clinical contract research services business, expanding its drug development and non-clinical research offerings.
- In July 2020, Syneos Health and Medable announced a strategic nership to optimize decentralized clinical trials using digital and mobile technologies.
- In June 2020, Charles River Laboratories International Inc. acquired Retrogenix Limited, strengthening its ability to provide comprehensive early-stage drug discovery services.
- In May 2020, Covance launched Covance Market Access, a dedicated service to support market access and reimbursement strategies for pharmaceutical and biotech companies.
- In March 2020, PPD nered with Science 37 to accelerate decentralized clinical trials, leveraging Science 37's technology platform.
- In January 2020, Medpace Holdings Inc. acquired the clinical research services business from Novella Clinical, expanding its global capabilities in clinical research.
- In December 2019, Pharmaceutical Product Development LLC (PPD) announced a collaboration with Science Exchange to provide researchers access to PPD’s analytical and biomarker services.
- In October 2019, ICON plc acquired Symphony Clinical Research, a leading provider of at-home and alternate-site clinical trial services.
Significant Growth Factors:
Factors contributing to the expansion of the healthcare contract research organization (CRO) sector comprise the rise in clinical trials, a surge in the need for specialized services, and the integration of cutting-edge technologies.
The healthcare Contract Research Organization (CRO) market is poised for substantial growth in the foreseeable future due to various driving factors. Key among these is the uptick in clinical trials carried out by pharmaceutical and biopharmaceutical entities, necessitating the services of CROs. This surge is propelled by the escalating prevalence of chronic illnesses, which has spurred extensive research and development endeavors in pursuit of effective and safe treatments.
Moreover, the outsourcing of research and development functions by life sciences companies stands out as a pivotal growth catalyst for the healthcare CRO sector. By tapping into the expertise, infrastructure, and global reach of CROs, businesses in the pharmaceutical realm can streamline drug development processes, cutting costs and time expenditures while honing in on core competencies.
Noteworthy is the mounting complexity of clinical trials, which demands specialized knowledge and cutting-edge technologies, thereby intensifying the reliance on CROs. These organizations provide a suite of comprehensive services spanning patient recruitment, data management, regulatory affairs, and biostatistics, ensuring the smooth and effective execution of trials.
Furthermore, the upsurge in personalized medicine and the emergence of targeted therapies have spurred a surge in demand for CRO services. CROs play a vital role in orchestrating specialized trials involving genetic profiling, biomarker identification, and companion diagnostics, thus fostering advancements in drug development efficiency.
The burgeoning pharmaceutical and biopharmaceutical industries in emerging markets like China and India offer a ripe landscape for growth opportunities for CROs. A large patient base, cost efficiencies, and supportive regulatory environments in these regions have attracted pharmaceutical stakeholders to engage in clinical trial outsourcing.
To summarize, the healthcare CRO market's growth is underpinned by an array of factors including the uptick in clinical trials, the outsourcing trend in research and
Key Segments of the Healthcare CRO Market
By Type
• Drug Discovery
• Pre-Clinical
• Clinical
By Service
• Project Management/Clinical Supply Management
• Data Management
• Regulatory/Medical Affairs
• Medical Writing
• Clinical Monitoring
• Quality Management/Assurance
• Bio-statistics
• Investigator Payments
• Laboratory
• Patient And Site Recruitment
• Technology
• Others
By Therapeutic Area
• Oncology
• Cardiovascular
• Infectious Diseases
• Neurology
By End-User
• Pharmaceutical Industries
• Biotech Industries
• Medical Device Industries
• Academic Institutions
Regional Overview
North America
• US
• Canada
• Mexico
Europe
• Germany
• France
• U.K
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Rest of Asia Pacific
Middle East and Africa
• Saudi Arabia
• UAE
• Rest of Middle East and Africa
Latin America
• Brazil
• Argentina
• Rest of Latin America

