Market Analysis and Insights:
The market for Global Enteral Feeding Devices Market was estimated to be worth USD 6.76 billion in 2022, and from 2023 to 2032, it is anticipated to grow at a CAGR of 6.53%, with an expected value of USD 12.62 billion in 2032.
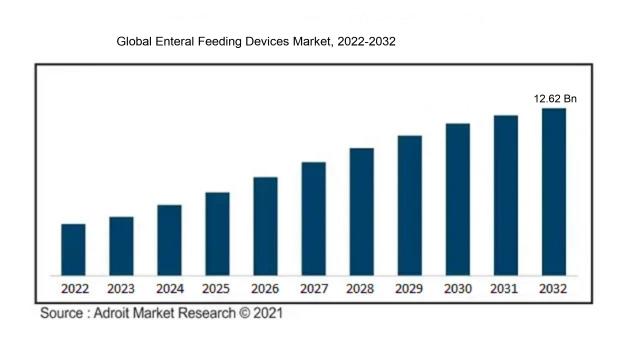
The Enteral Feeding Devices Market is experiencing notable growth due to several key factors. The escalating prevalence of chronic ailments like cancer, diabetes, and gastrointestinal disorders necessitating prolonged nutritional support is a significant driver. Another contributing factor is the expanding elderly population and the increasing number of preterm births, as these groups are more susceptible to malnutrition and often require enteral feeding for their dietary requirements. Market growth is further fueled by technological advancements such as the emergence of smart pumps and specialized feeding tubes, which enhance the safety and efficiency of enteral feeding procedures. The trend of adopting enteral nutrition in homecare environments, driven by the demand for cost-effective and patient-centric care, is also fostering market expansion. Additionally, favorable reimbursement policies, government initiatives to promote awareness about enteral nutrition, and the rising preference for minimally invasive procedures are further propelling market growth. Despite these driving factors, challenges such as the potential risks of infections and complications associated with enteral feeding may pose hindrances to market progression. Nevertheless, the market is anticipated to sustain growth in the foreseeable future owing to these influential factors.
Enteral Feeding Devices Market Scope :
| Metrics | Details |
| Base Year | 2023 |
| Historic Data | 2018-2022 |
| Forecast Period | 2024-2032 |
| Study Period | 2018-2032 |
| Forecast Unit | Value (USD) |
| Revenue forecast in 2032 | USD 12.62 billion |
| Growth Rate | CAGR of 6.53% during 2023-2032 |
| Segment Covered | By Product,By Age Group,By Indication,By End-use,By Region. |
| Regions Covered | North America, Europe, Asia Pacific, South America, Middle East and Africa |
| Key Players Profiled | encompass Medtronic, Abbott Laboratories, Fresenius Kabi AG, Cook Medical, C.R. Bard Inc., Danone Nutricia, Moog Inc., B. Braun Melsungen AG, Nestle Health Science, and Boston Scientific Corporation. |
Market Definition
The enteral feeding equipment industry pertains to the worldwide domain dedicated to manufacturing and supplying medical tools designed for delivering nutrition via the gastrointestinal system. These tools are predominantly employed for individuals who have difficulties with oral food intake.
The significance of the Enteral Feeding Devices Market stems from the rise in chronic illnesses like cancer, diabetes, and neurological conditions, which often lead to difficulties with swallowing or inadequate nutritional consumption.
These devices are essential for delivering vital nutrients and medications directly to patients' digestive systems, ensuring they receive proper nourishment and enhancing their overall health. Moreover, enteral feeding devices facilitate at-home enteral nutrition, offering convenient and cost-efficient choices for long-term care. The market's importance lies in its capacity to meet the escalating demand for these devices and assist the healthcare sector in addressing patients' nutritional requirements, thereby enhancing their quality of life.
Key Market Segmentation:

Insights On Key Product
Percutaneous Endoscopic Gastrostomy Device
Percutaneous Endoscopic Gastrostomy (PEG) devices are expected to dominate the Global Enteral Feeding Devices Market. PEG devices are widely used for long-term enteral feeding and provide a safer and more effective alternative to traditional methods such as nasogastric tubes. The growing prevalence of chronic diseases, increasing geriatric population, and rising demand for minimally invasive procedures are driving the demand for PEG devices. Additionally, advancements in technology, such as the development of low-profile gastrostomy devices, have further enhanced the adoption of PEG devices. With their proven efficacy and benefits, PEG devices are likely to continue dominating the global enteral feeding devices market in the foreseeable future.
Giving Set
Giving sets play a crucial role in enteral feeding by connecting the enteral feeding pump to the feeding tube. While giving sets are an essential component of the overall enteral feeding process, they are not expected to dominate the market. The demand for giving sets largely depends on the adoption of enteral feeding pumps and other feeding devices. Therefore, the growth of the giving sets will be driven by the demand for other dominant s like enteral feeding pumps and PEG devices.
Enteral Feeding Pump
Enteral feeding pumps are designed to accurately deliver enteral nutrition to patients. While enteral feeding pumps are an important of enteral feeding, they are not expected to dominate the market. The dominance is more likely to be determined by the demand for PEG devices, which serve as the primary means of long-term enteral feeding. However, the demand for enteral feeding pumps will continue to grow as they offer benefits such as accurate and controlled feeding, ease of use, and portability.
Low Profile Gastrostomy Device
Low profile gastrostomy devices are a type of PEG device that offers a more discreet and comfortable option for patients. While their popularity is increasing, they are not expected to dominate the market. Low profile gastrostomy devices are a niche within the larger PEG devices market. The preference for low profile gastrostomy devices may vary among patients and healthcare providers, but their overall market share is likely to remain lower than the standard PEG devices.
Nasogastric Tube
Nasogastric tubes are temporary feeding tubes inserted through the nose and down to the stomach. They are widely used in short-term enteral feeding or for diagnostic and therapeutic purposes. However, nasogastric tubes are not expected to dominate the market for enteral feeding devices. The growing popularity of PEG devices, which offer long-term feeding solutions with lower risks and improved patient comfort, limits the potential dominance of nasogastric tubes in the global market.
Gastrostomy Tube
Gastrostomy tubes are used to provide direct access to the stomach for enteral feeding. While gastrostomy tubes are an important of the enteral feeding devices market, they are not expected to dominate the market. The dominance is more likely to be determined by the demand for PEG devices, which offer advantages such as easier insertion, reduced risk of complications, and better long-term management. The gastrostomy tube will continue to have its importance, especially for specific patient populations or clinical scenarios, but it is not expected to dominate the market compared to PEG devices.
Insights On Key Age Group
Adults
The adults is expected to dominate the Global Enteral Feeding Devices Market. This can be attributed to the larger patient population of adults requiring enteral feeding due to various medical conditions such as gastrointestinal disorders, cancer, and neurological disorders. Furthermore, the increasing prevalence of chronic illnesses and the growing aging population contribute to the higher demand for enteral feeding devices among adults. Additionally, advancements in technology have led to the development of innovative and user-friendly devices, catering to the specific needs of adult patients.
Pediatrics
In contrast to the adults , the pediatrics is not expected to dominate the Global Enteral Feeding Devices Market. Although there is a significant demand for enteral feeding devices among pediatric patients, the market is relatively smaller compared to the adults . This can be attributed to the lower incidence of chronic illnesses requiring enteral feeding in children, as well as the challenges associated with designing and developing devices suitable for pediatric use. However, advancements in pediatric enteral feeding devices and an increasing awareness of the benefits of enteral nutrition in pediatric patient care are expected to drive the growth of this in the coming years.
Insights On Key Indication
Cancer Care
Among the various s of By Indication, Cancer Care is expected to dominate the Global Enteral Feeding Devices Market. The increasing prevalence of cancer worldwide, coupled with the growing number of patients undergoing cancer treatment, has led to a significant demand for enteral feeding devices in this indication. Cancer patients often experience difficulty in eating and maintaining proper nutrition due to the side effects of treatment, such as nausea, vomiting, and mouth sores. Enteral feeding devices provide a safe and effective way to deliver essential nutrients and fluids directly into the patients' digestive system, ensuring proper nourishment and promoting recovery. As a result, the demand for enteral feeding devices in the Cancer Care is projected to be high.
Alzheimer's
In the By Indication category, Alzheimer's is a with significant potential in the Global Enteral Feeding Devices Market. Alzheimer's disease is a progressive neurological disorder that affects memory, cognition, and behavior. As the disease advances, individuals with Alzheimer's often experience difficulty swallowing and may require enteral nutrition for their nutritional needs. The rising prevalence of Alzheimer's disease globally, along with the increasing aging population, is expected to drive the demand for enteral feeding devices in this .
Diabetes
The Diabetes of By Indication holds considerable potential in the Global Enteral Feeding Devices Market. Diabetes is a chronic metabolic disorder characterized by high blood sugar levels. Individuals with uncontrolled diabetes may develop complications such as gastroparesis, a condition that affects the stomach's ability to empty properly. Enteral feeding devices can help manage the nutritional needs of patients with gastroparesis by delivering nutrients directly to the small intestine. The growing prevalence of diabetes worldwide, fueled by factors like sedentary lifestyles and unhealthy eating habits, is expected to drive the demand for enteral feeding devices in this .
Chronic Kidney Diseases
The Chronic Kidney Diseases of By Indication is expected to have a significant impact on the Global Enteral Feeding Devices Market. Chronic kidney disease (CKD) is a long-term condition characterized by the gradual loss of kidney function. Patients with advanced CKD may experience poor appetite, unintentional weight loss, and other nutritional challenges. Enteral feeding devices can help provide adequate nutrition to CKD patients, ensuring they receive essential nutrients while managing their fluid and electrolyte balance. The increasing prevalence of CKD globally, driven by factors such as aging populations and the rising incidence of hypertension and diabetes, is expected to fuel the demand for enteral feeding devices in this .
Nutrition Deficiency
Nutrition Deficiency is a vital in the By Indication category of the Global Enteral Feeding Devices Market. Despite advances in healthcare, malnutrition remains a significant public health issue worldwide. Many individuals suffer from nutrient deficiencies due to factors such as inadequate dietary intake, chronic illnesses, or impaired nutrient absorption. Enteral feeding devices play a crucial role in addressing nutrition deficiencies by providing targeted and controlled delivery of essential nutrients. The rising awareness about the importance of proper nutrition and the increasing prevalence of malnutrition in different populations are expected to drive the demand for enteral feeding devices in this .
Orphan Diseases
Orphan Diseases are a significant within By Indication, contributing to the Global Enteral Feeding Devices Market. Orphan diseases, also referred to as rare diseases, are medical conditions that affect a small population. These diseases often lead to various health complications, including nutritional deficiencies and difficulty swallowing. Enteral feeding devices offer a viable solution to address the specific nutrition requirements of individuals with orphan diseases, ensuring they receive adequate nourishment. The growing recognition and focus on rare diseases by healthcare organizations and government initiatives worldwide are expected to drive the demand for enteral feeding devices in this .
Dysphagia
The Dysphagia within By Indication holds significance in the Global Enteral Feeding Devices Market. Dysphagia is a condition characterized by difficulty swallowing, which can arise due to neurological disorders, stroke, or structural abnormalities in the throat or esophagus. Individuals with dysphagia often require alternate means of nutrition, such as enteral feeding, to ensure safe and adequate nutritional intake. The increasing prevalence of dysphagia, driven by factors such as an aging population and the rising incidence of neurological disorders, is expected to contribute to the demand for enteral feeding devices in this .
Pain Management
Pain management is an important within By Indication that has an impact on the Global Enteral Feeding Devices Market. Patients undergoing pain management treatment, icularly those receiving long-term opioid therapy, may experience incidental bowel dysfunction, such as constipation. Enteral feeding devices can be used to optimize hydration and nutrition while managing opioid-induced gastrointestinal side effects. The growing demand for pain management therapies and an increased focus on managing opioid-related complications are expected to drive the usage of enteral feeding devices in this .
Malabsorption/GI Disorder/Diarrhea
The Malabsorption/GI Disorder/Diarrhea within By Indication plays a significant role in the Global Enteral Feeding Devices Market. Malabsorption, gastrointestinal disorders, and diarrhea are conditions that can hinder proper nutrient absorption and digestion. Enteral feeding devices enable the controlled and targeted delivery of nutrients, ensuring that patients with these disorders receive optimal nutrition. The increasing prevalence of malabsorption, gastrointestinal disorders, and diarrhea globally, due to factors such as infections, inflammatory bowel diseases, and irritable bowel syndrome, is expected to drive the demand for enteral feeding devices in this .
Others
The Other within By Indication encompasses various indications that do not fall under the dominant category in the Global Enteral Feeding Devices Market. It includes a range of indications with varying levels of market potential. The demand for enteral feeding devices in this depends on the prevalence and specific requirements of each indication. Factors such as disease prevalence, patient population, and the availability of alternative treatment options will influence the demand for enteral feeding devices across each individual indication within this .
Insights On Key End-use
Hospitals
Hospitals are expected to dominate the Global Enteral Feeding Devices Market. This is due to several factors. Firstly, hospitals have a higher patient volume compared to home care settings, leading to a greater demand for enteral feeding devices. Additionally, hospitals typically have specialized de ments and healthcare professionals who are trained in managing enteral nutrition, further driving the usage of these devices. Moreover, hospitals often treat patients with more complex medical conditions, who may require enteral feeding for a longer duration, creating a continuous demand for enteral feeding devices within hospital settings.
Home Care
In contrast to hospitals, the Home Care is not expected to dominate the Global Enteral Feeding Devices Market. Home care settings primarily cater to patients who can receive care in the comfort of their own homes, including those recovering from surgery or managing chronic illnesses. While enteral feeding devices may still be utilized in home care, the patient population and usage rate are generally lower compared to hospitals. Therefore, the demand and market share for enteral feeding devices in home care settings are expected to be smaller compared to the dominating hospital .
Insights on Regional Analysis:
North America
North America is expected to dominate the Global Enteral Feeding Devices Market. This can be attributed to several factors such as the high prevalence of chronic diseases, high healthcare expenditure, technological advancements, and well-established healthcare infrastructure in the region. Moreover, the increasing geriatric population and the rising demand for home enteral nutrition solutions are further contributing to the growth of the market in this region. Additionally, advancements in healthcare reimbursement policies and the presence of key market players in North America are driving the market growth.
Latin America
Latin America is projected to witness significant growth in the Global Enteral Feeding Devices Market. This can be attributed to the increasing awareness about enteral nutrition, rising prevalence of chronic diseases, and improving healthcare infrastructure in the region. Moreover, the growing geriatric population and the increasing adoption of enteral feeding devices in home healthcare settings are further fueling the market growth in Latin America. However, economic and political instability in some countries, along with the lack of reimbursement policies, may hinder the market growth to some extent.
Asia Pacific
Asia Pacific is expected to witness substantial growth in the Global Enteral Feeding Devices Market. This can be attributed to factors such as the rising geriatric population, increasing prevalence of chronic diseases, and improving healthcare infrastructure in the region. Moreover, the growth of medical tourism, increasing healthcare expenditure, and the presence of a large patient pool are driving the market growth in Asia Pacific. However, challenges such as the lack of awareness about enteral nutrition, limited healthcare access in rural areas, and variations in reimbursement policies may hinder the market growth in some countries within the region.
Europe
Europe is expected to have a significant share in the Global Enteral Feeding Devices Market. This can be attributed to several factors such as the presence of well-established healthcare infrastructure, increasing prevalence of chronic diseases, and advancements in medical technology. Moreover, the rising geriatric population and the growing adoption of enteral feeding devices in home healthcare settings contribute to the market growth in Europe. Additionally, favorable reimbursement policies and the presence of major market players in the region are supporting the market expansion in Europe.
Middle East & Africa
The Middle East & Africa region is expected to witness moderate growth in the Global Enteral Feeding Devices Market. This can be attributed to factors such as the increasing prevalence of chronic diseases and the growing awareness about enteral nutrition in the region. Moreover, the rise in healthcare expenditure and the improving healthcare infrastructure are contributing to the market growth. However, challenges such as the limited access to advanced medical devices in certain regions, economic constraints, and variations in healthcare policies may limit the market growth in the Middle East & Africa.
Company Profiles:
Prominent participants in the worldwide enteral feeding devices industry are tasked with the production and distribution of medical tools utilized for enteral feeding administration. They have a crucial responsibility in addressing the increasing market need for such devices while upholding standards of excellence and fostering innovation.
The key stakeholders in the Enteral Feeding Devices industry encompass Medtronic, Abbott Laboratories, Fresenius Kabi AG, Cook Medical, C.R. Bard Inc., Danone Nutricia, Moog Inc., B. Braun Melsungen AG, Nestle Health Science, and Boston Scientific Corporation. These organizations are prominent figures within the sector and are actively engaged in the research, production, and dissemination of enteral feeding devices. Their commitment to innovation drives the introduction of cutting-edge products to meet the escalating demand for enteral feeding solutions. Fierce competition characterizes the interactions among these primary actors as they vie for market dominance and seek to broaden their footprint in the global Enteral Feeding Devices arena. Furthermore, these entities ake in strategic alliances, collaborations, and mergers and acquisitions to fortify their market standing and secure a competitive advantage.
COVID-19 Impact and Market Status:
The worldwide enteral feeding devices industry has experienced a notable impact from the Covid-19 pandemic, resulting in a deceleration in expansion attributed to interruptions in the healthcare infrastructure and supply chain.
The enteral feeding devices market has been substantially impacted by the COVID-19 pandemic. As healthcare resources have been primarily directed towards managing COVID-19 cases, there has been a noticeable decrease in the demand for enteral feeding devices. Numerous elective medical procedures were postponed, leading to a reduced requirement for these devices. Furthermore, disruptions in the supply chain due to lockdown restrictions have hampered the manufacturing and distribution of enteral feeding devices. Consequently, hospitals and healthcare institutions have encountered difficulties in acquiring and maintaining sufficient stocks of these devices. Conversely, the escalating hospitalization rates of COVID-19 patients have resulted in a ened need for enteral feeding devices in critical care units. The necessity to provide nutrition to patients facing severe respiratory challenges has propelled the demand for these devices. In summary, the enteral feeding devices market has faced a blend of obstacles and prospects as a result of the COVID-19 crisis, and the lasting effects will depend on the pandemic's duration and severity.
Latest Trends and Innovation:
- In January 2020, Boston Scientific Corporation announced the acquisition of Vertiflex, Inc., a company specializing in minimally invasive treatments for patients suffering from lumbar spinal stenosis.
- In July 2020, Fresenius Kabi AG completed the acquisition of Merck KGaA’s biosimilars business, strengthening its position in the global healthcare market.
- In November 2020, Medtronic plc launched the ENCHANT™ Catheter, a device designed for enteral feeding in neonatal intensive care units (NICUs) to meet the unique needs of premature infants.
- In March 2021, Abbott Laboratories acquired GlucoSet AS, a company focused on developing and commercializing continuous glucose monitoring systems for use in hospitals.
- In May 2021, B. Braun Melsungen AG introduced the Space GlucoseControl system, an integrated solution for managing blood glucose levels in critically ill patients receiving enteral nutrition.
- In August 2021, Cardinal Health, Inc. announced the acquisition of Medline Industries, Inc., a global manufacturer and distributor of medical and surgical products, enhancing Cardinal Health's offerings in enteral feeding devices and other healthcare sectors.
Significant Growth Factors:
The expansion catalysts propelling the Enteral Feeding Devices Industry comprise the escalating incidence of chronic illnesses and aging demographic, growing preference for home enteral nutrition, and advancements in technology related to enteral feeding devices.
The market for enteral feeding devices is witnessing substantial growth for various reasons. The increasing prevalence of chronic diseases like cancer, neurological disorders, and gastrointestinal conditions has fueled the demand for such devices. Additionally, the expanding elderly population, who are more susceptible to these illnesses, is a key driver behind the rising need for enteral feeding devices. Furthermore, advancements in technology have enhanced the safety and usability of these devices, making them more appealing to healthcare professionals and patients alike. The growing awareness of the advantages of enteral nutrition and the preference for home-based enteral feeding have also contributed to market expansion. Moreover, the augmented healthcare spending in developing nations and the enhancement of healthcare infrastructure are further propelling market growth. Government and healthcare insurance reimbursement policies are also facilitating the uptake of enteral feeding devices. Collaborations and nerships between manufacturers and healthcare institutions for the development and commercialization of products are bolstering market expansion. Nevertheless, challenges like the high costs of enteral feeding devices and associated usage complications may pose hindrances to market growth. However, ongoing research and development efforts aimed at improving the safety and effectiveness of these devices are anticipated to create lucrative opportunities for growth in the foreseeable future.
Restraining Factors:
The enteral feeding devices sector could encounter obstacles related to regulatory hurdles and potential unfavorable incidents linked to the utilization of such equipment.
The market for enteral feeding devices is currently facing various challenges that are impeding its potential for expansion. A primary obstacle lies in the elevated costs associated with these devices, rendering them less accessible to individuals with limited financial resources. Furthermore, a lack of awareness among healthcare providers and patients regarding the advantages and correct utilization of such devices presents a substantial barrier. Additionally, the scarcity of proficient healthcare professionals proficient in the management of enteral feeding devices results in suboptimal patient care. Moreover, stringent regulatory mandates and the necessity for substantial clinical substantiation for device endorsement impede the timely introduction of new and innovative products to the market. Concerns regarding complications such as tube dislodgement, aspiration, and infections linked to enteral feeding devices contribute to safety apprehensions among both healthcare providers and patients. Furthermore, inadequate reimbursement coverage in certain regions restricts the adoption of enteral feeding devices and constrains market growth. Nonetheless, amidst these challenges, factors like the rising incidence of chronic diseases, an aging population, and advancements in device technology present growth opportunities for the market. To surmount these obstacles in the long term, it will be crucial to focus on increasing awareness, enhancing cost-efficiency, bolstering training initiatives for healthcare professionals, and streamlining regulatory procedures.
Key Segments of the Enteral Feeding Devices Market
Product Overview
• Giving Set
• Enteral Feeding Pump
• Percutaneous Endoscopic Gastrostomy Device
• Low Profile Gastrostomy Device
• Nasogastric Tube
• Gastrostomy Tube
Age Group Overview
• Adults
• Pediatrics
Indication Overview
• Alzheimer’s
• Nutrition Deficiency
• Cancer Care
• Diabetes
• Chronic Kidney Diseases
• Orphan Diseases
• Dysphagia
• Pain Management
• Malabsorption/GI Disorder/Diarrhea
• Others
End-Use Overview
• Hospitals
• Home Care
Regional Overview
North America
• US
• Canada
• Mexico
Europe
• Germany
• France
• U.K
• Rest of Europe
Asia Pacific
• China
• Japan
• India
• Rest of Asia Pacific
Middle East and Africa
• Saudi Arabia
• UAE
• Rest of Middle East and Africa
Latin America
• Brazil
• Argentina
• Rest of Latin America

