Controlled Release Drug Delivery Market Analysis and Insights:
In 2023, the size of the worldwide Controlled Release Drug Delivery market was US$ 88.94 billion. Adroit Market Research projects that the market will increase at a compound annual growth rate (CAGR) of 9.36% from 2024 to 2032, reaching US$ 141.06 billion.
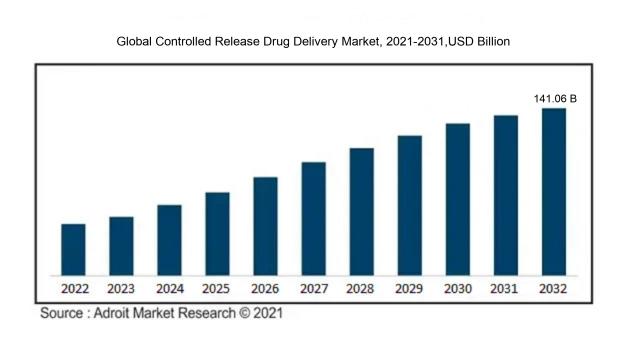
The market for Controlled Release Drug Delivery is significantly influenced by progress in pharmaceutical technologies that improve both the effectiveness and safety of medications. The demand for enhanced therapeutic results and greater patient adherence is driving innovation in drug formulations that utilize sustained release methods. Moreover, the rising incidence of chronic illnesses, including diabetes and cardiovascular diseases, underscores the need for more effective long-term treatment alternatives. Increased research and development efforts focused on innovative drug delivery methods, such as those employing nanoparticles and polymer-based technologies, are key factors in advancing market growth. Support from regulatory agencies for controlled release products, combined with a growing emphasis on personalized medicine, plays a critical role as well. Additionally, demographic changes, particularly the aging population, and a trend towards injectable and implantable delivery systems are facilitating market growth, as these methods enhance dosing precision and minimize side effects, ultimately improving patient treatment experiences.
Controlled Release Drug Delivery Market Definition
Controlled release drug delivery is an innovative approach for administering medication that facilitates a gradual release of the drug over a defined timeframe, ensuring consistent therapeutic levels in the bloodstream. This technique not only boosts effectiveness but also reduces adverse effects and enhances patient adherence by decreasing the number of doses needed.
The technology of controlled release drug delivery plays a vital role in enhancing therapeutic effectiveness by preserving stable drug concentrations in the bloodstream for prolonged durations. This approach mitigates the fluctuations typically seen with standard delivery methods. It enables precise drug targeting, which helps decrease adverse effects and boosts patient adherence due to the reduced frequency of medication administration. Moreover, controlled release mechanisms can improve drug absorption and bioavailability, providing substantial benefits for the management of chronic illnesses. By customizing release kinetics, these systems support personalized treatment plans, guaranteeing that patients receive the optimal dose at the appropriate intervals, which is crucial for achieving the best therapeutic results and elevating overall health quality.
Controlled Release Drug Delivery Market Segmental Analysis:

Insights On Technology
Targeted Delivery
Targeted delivery is anticipated to dominate the Global Controlled Release Drug Delivery Market due to its potential for enhancing therapeutic effectiveness while minimizing side effects. This technology specifically directs drugs to particular sites in the body, thereby increasing the concentration of the drug at the intended target and improving patient outcomes. The growing prevalence of chronic diseases, coupled with the rising demand for personalized medicine, has significantly boosted investment in targeted delivery systems. Advancements in nanotechnology and biopharmaceuticals further catalyze its adoption, making it the preferred choice among researchers and healthcare professionals aiming for precision in drug administration.
Coacervation
Coacervation has gained traction in the controlled release drug delivery arena due to its ability to form stable microcapsules that encapsulate therapeutic agents effectively. This method allows for the creation of particles with tunable release profiles, enabling a controlled and sustained release of drugs over time. Coacervation is adaptable and can be used for a variety of purposes, such as injectable and oral versions. As interest in improving patient adherence to treatment regimens rises, coacervation's potential to enhance the bioavailability of drugs makes it a notable contender in the market.
Implants
Implants offer a unique approach to controlled drug delivery, enabling long-term therapeutic administration without the need for frequent dosing. This approach is particularly helpful for long-term diseases that need for steady dosages of medication. Implants can be designed to degrade within the body or remain indefinitely, depending on the therapeutic need. Implant technology is gaining popularity due to a number of factors, including the rising incidence of chronic illnesses and patient preferences for less invasive delivery systems. This sustained release capability is particularly critical in pain management and hormonal therapies.
Micro Encapsulation
Micro encapsulation is a vital technique in controlled release drug delivery, allowing drug substances to be enclosed within a carrier matrix. This process not only protects sensitive compounds from degradation but also enables the release of drugs in a controlled manner. The advantage of micro encapsulation lies in its versatility; it can be applied to various drug types, including proteins and vaccines. As the demand for innovative formulations and enhanced drug stability rises, micro encapsulation continues to attract attention, making it an essential part of the controlled release landscape.
Transdermal
Transdermal technology facilitates drug delivery through the skin, offering a non-invasive mechanism for sustained release. Without requiring injections or oral ingestion, this technique improves drug absorption and sustains therapeutic levels. The convenience and patient compliance associated with transdermal patches make them increasingly popular for treatments ranging from hormone replacement to pain management. As research progresses in optimizing skin penetration enhancers and formulation techniques, the transdermal drug delivery is expected to witness continued growth within the overall controlled release market.
Wurster Technique
The Wurster Technique is recognized for its effectiveness in producing controlled-release formulations through fluid bed coating processes. This technology utilizes polymeric coatings that allow drugs to be released gradually, thereby improving dosing intervals and patient adherence. Its application is especially beneficial for different oral pharmaceutical forms, such as granules and tablets. With increasing demand for precise dose control and the ability to optimize drug release profiles, the Wurster Technique remains a relevant technology in the realm of controlled release drug delivery systems.
Others (Liposomes, Microelectromechanical Technology)
The category encompassing liposomes and microelectromechanical technology has emerged as an innovative frontier in controlled release drug delivery. Liposomes serve as versatile carriers that can improve drug solubility, stability, and targeted delivery. As advancements in biotechnology continue, the use of liposomes for delivering chemotherapeutics and vaccines is expanding. Meanwhile, microelectromechanical technology offers integration of drug delivery with miniaturized devices, promising future innovations in precision medicine and real-time monitoring of drug release. This category's dynamism is a reflection of the ongoing research and technological advancements transforming the drug delivery landscape.
Insights On Release Mechanism
Drug Delivery Systems that are feedback regulated
Drug Delivery Systems that are feedback regulated are expected to dominate the Global Controlled Release Drug Delivery Market as they typically focus on passive drug release and have been employed for decades in various therapeutic applications. While they offer simplicity and ease of use, their potential for controlled release is generally limited compared to newer modalities. The shift towards personalized medicine and precision therapy is driving innovation in drug delivery techniques, putting these conventional systems at a disadvantage as the market increasingly favors more sophisticated options that can efficiently meet patient needs over prolonged periods.
Systems Based on Polymers
Polymer Based Systems are expected to dominate the worldwide controlled release drug delivery market among the several release mechanisms. These systems offer significant advantages in terms of versatility, biocompatibility, and the ability to customize drug release profiles according to specific therapeutic needs. The growing demand for advanced therapies, particularly in chronic disease management and oncology, has fueled the expansion of polymer-based formulations. Moreover, advancements in polymer science have led to the development of innovative materials that enhance drug encapsulation and control release dynamics, making them a favored choice for researchers and pharmaceutical developers.
Partition Controlled Micro Reservoir Drug Delivery Systems
Partition Controlled Micro Reservoir Drug Delivery Systems are characterized by their ability to control drug release through diffusion processes across a polymeric membrane. These systems are effective for delivering small molecules over extended periods, which is beneficial in scenarios requiring consistent therapeutic levels. However, despite their advantages, they face competition from more advanced polymer-based systems, which offer greater flexibility and scalability in production, thus limiting their market penetration.
Activation-modulated Drug Delivery Systems
Activation-modulated Drug Delivery Systems utilize external stimuli to control drug release, encompassing methods like hydrodynamic, osmotic, and magnetically activated mechanisms. Although these systems show promise, their complexity and sometimes higher fabrication costs can restrict widespread adoption. Furthermore, the market trend leans towards simpler, user-friendly systems that can be easily integrated into standard treatment protocols, which may hinder the growth of activation-modulated systems compared to polymer-based solutions.
Chemically Activated Drug Delivery Systems
Chemically Activated Drug Delivery Systems leverage biochemical reactions such as hydrolysis, pH changes, and enzymatic activity to achieve targeted drug release. While these systems can offer precise control and site-specific delivery, they often require intricate formulation strategies and stability considerations, which can complicate manufacturing processes. Consequently, the increasing focus on practicality and efficiency in drug delivery is overshadowing the potential of chemically activated systems in a competitive market landscape dominated by polymer-based innovations.
Insights On Application
Oral Controlled-drug Delivery Systems
Oral controlled-drug delivery systems are expected to dominate due to their ease of administration, patient compliance, and ability to provide a steady drug release profile. The market has shown a significant inclination toward formulations that allow for sustained therapeutic effects with fewer side effects. Patients prefer oral routes over injections or infusions, which is crucial for chronic disease management, leading to increased demand for these systems. Additionally, advancements in formulation technology, such as polymers and nanotechnology, are enhancing the efficacy and efficiency of oral delivery systems, thus securing their position as the leading application category in this market.
Injectable Metered Dose Inhalers
Injectable metered dose inhalers are gaining traction in the controlled release drug delivery landscape, particularly for pulmonary diseases where rapid and effective drug delivery is critical. These devices allow for portability and precise dosing, making them ideal for asthma and COPD management. The innovation in inhalation technology is contributing to more effective drug formulations that enhance bioavailability and reduce side effects. This steady evolution makes them a sought-after choice but still remains secondary in the overall market.
Infusion Pumps
Infusion pumps are well-established systems in the landscape of controlled release drug delivery, particularly in hospital settings for the administration of chemotherapy and pain management. Their capability of delivering continuous subcutaneous medication allows for better therapeutic control. Recent developments in smart infusion technology and integrated devices have significantly improved the safety and efficiency of drug delivery, especially in critical care contexts. However, their complexity and the requirement for clinical oversight limit their market share compared to oral forms.
Ocular and Transdermal Patches
Ocular and transdermal patches offer a unique and patient-friendly method for drug delivery, especially for localized treatments and conditions that require minimal invasiveness. Their ability to bypass the gastrointestinal tract and hepatic first-pass metabolism allows for enhanced drug bioavailability. Despite their advantages, the uptake in the market is moderate when placed next to oral controlled-drug systems, as they are more suited for specific conditions rather than general use.
Eluting Drug Stents
Eluting drug stents play a critical role in the management of cardiovascular diseases by releasing anti-proliferative or anti-clotting drugs directly at the site of vascular intervention. Their localized delivery mechanism significantly improves treatment outcomes and reduces the risk of restenosis. While they represent a specialized application category focusing on interventional cardiology, their market presence is narrower compared to commonly used delivery forms like oral medications, limiting their overall impact on the broader controlled release drug delivery market.
Global Controlled Release Drug Delivery Market Regional Insights:
North America
The global market for controlled release drug delivery is anticipated to be dominated by North America. This is caused by a number of elements, such as the existence of major pharmaceutical firms, a strong regulatory environment, and large R&D expenditures. The area is renowned for its cutting-edge medical facilities, easy access to medical specialists, and growing investment in cutting-edge medication delivery technologies. Furthermore, the need for controlled release formulations is driven by the rising incidence of chronic illnesses that necessitate long-term pharmaceutical maintenance. Additionally, North America's focus on personalized medicine and technological advancements in drug delivery systems further solidify its position as a leader in this market.
Latin America
Because of rising healthcare costs and the prevalence of chronic diseases, Latin America exhibits considerable growth potential in the global market for controlled release drug delivery. Important considerations include the region's growing pharmaceutical industry and initiatives to improve access to healthcare. Furthermore, government initiatives to improve healthcare systems and collaboration with global pharmaceutical companies to innovate drug delivery solutions will likely drive market expansion. However, infrastructural challenges and regulatory issues may hinder swift growth.
Asia Pacific
Asia Pacific is on a growth trajectory in the Global Controlled Release Drug Delivery market, primarily driven by an expanding population and burgeoning healthcare needs. Advanced medication delivery technologies are being developed as a result of significant investments made in healthcare systems by nations like China and India. Rising disposable incomes, increasing awareness about health management, and a booming pharmaceutical market contribute to this positive trend. Nevertheless, regional disparities in regulatory standards and healthcare infrastructure could pose challenges to the market's overall expansion.
Europe
Europe is witnessing gradual growth in the Global Controlled Release Drug Delivery market, fueled by an emphasis on research and innovation within its pharmaceutical sector. The presence of several long-standing pharmaceutical companies and strong regulatory bodies encourages the development of new drug delivery technologies. Furthermore, the European Medicines Agency (EMA) supports innovative solutions in drug delivery systems, promoting accessible treatments. However, competition among European countries and economic fluctuations can impact market stability and growth rates.
Middle East & Africa
The global market for controlled release drug delivery is growing slowly but steadily in the Middle East and Africa area. Progress is being fueled by elements including growing public spending on healthcare and growing knowledge of cutting-edge medical procedures. A conducive environment for growth is also produced by the region's initiatives to upgrade its healthcare infrastructure and draw in foreign pharmaceutical investments. However, challenges such as limited healthcare access and political instability can impede the rapid expansion of controlled drug delivery solutions within this region.
Controlled Release Drug Delivery Market Competitive Landscape:
Leading contributors in the worldwide controlled release drug delivery sector focus on developing sophisticated drug formulations and technologies that improve treatment effectiveness while reducing adverse effects. The major contributors to the Controlled Release Drug Delivery Market comprise Amgen Inc., Johnson & Johnson, Novartis AG, Pfizer Inc., Merck & Co., Inc., Roche Holding AG, AbbVie Inc., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Bayer AG, GlaxoSmithKline plc, Sanofi S.A., Bausch Health Companies Inc., Mallinckrodt Pharmaceuticals, and AstraZeneca PLC. Furthermore, influential entities such as Indivior PLC, Gilead Sciences, Inc., Hikma Pharmaceuticals PLC, Aquestive Therapeutics, Inc., and Sandoz (a subsidiary of Novartis) also have a prominent presence in this sector.
Global Controlled Release Drug Delivery COVID-19 Impact and Market Status:
The Covid-19 pandemic notably expedited advancements and the necessity for controlled release drug delivery systems, spurred by the pressing requirement for efficient treatments and vaccines to combat the virus.
The COVID-19 pandemic profoundly affected the Controlled Release Drug Delivery Market, initially causing interruptions in supply chains and postponing clinical trials and product launches due to lockdown measures and restrictions. Nevertheless, the crisis also fueled a ened demand for advanced drug delivery systems, particularly aimed at vaccines and therapeutics for COVID-19 and other conditions. The rise of telemedicine and an increased focus on home healthcare underscored the importance of patient-centric drug delivery solutions, fostering innovations within controlled release technologies. Furthermore, the pandemic underscored the necessity for more precise and efficient therapies, prompting amplified research and development efforts in this area. As healthcare systems continue to adapt to the persistent challenges brought about by COVID-19, the controlled release drug delivery market is projected to grow, supported by ened investments in pharmaceuticals and biotechnology, as well as an increasing focus on personalized medicine and the management of chronic diseases.
Latest Trends and Innovation in The Global Controlled Release Drug Delivery Market:
- In July 2022, Amgen announced the acquisition of Horizon Therapeutics for approximately $27.8 billion, significantly enhancing Amgen's pipeline with Horizon's innovative therapies focused on rare diseases, expanding their controlled release drug formulations.
- In September 2022, Emergent BioSolutions completed the acquisition of the biologics subsidiary of the UK-based contract development and manufacturing organization (CDMO) BioProducts Laboratory, bolstering their capabilities in controlled release drug development, particularly for vaccines and therapeutics.
- In March 2023, Gerresheimer AG unveiled a new generation of prefillable syringes designed for controlled drug release, allowing for more precise dosing and improved patient safety, targeting a significant market expansion in biologic drug delivery systems.
- In April 2023, Moderna entered into a collaboration with Arcturus Therapeutics focusing on the development of mRNA vaccines utilizing controlled release technologies, indicating moves toward more efficient delivery mechanisms for prolonged immunity effects
- In June 2023, Pfizer announced a strategic partnership with NanoString Technologies to develop personalized controlled release drug delivery systems leveraging advanced nanotechnology platforms to improve therapeutic efficacy for oncology patients.
- In August 2023, Johnson & Johnson's Janssen division received FDA approval for a long-acting injectable formulation of paliperidone, implementing controlled release technology to enhance medication adherence in patients with schizophrenia.
- In October 2023, Bayer AG introduced a new polymer-based controlled release platform that allows for the sustained delivery of therapeutic agents over extended periods, marking a major advancement in their drug development strategies in chronic disease management.
Controlled Release Drug Delivery Market Growth Factors:
The expansion of the Controlled Release Drug Delivery Market is propelled by innovations in pharmaceutical technologies, a rise in the incidence of chronic illnesses, and the necessity for enhanced adherence among patients.
The Controlled Release Drug Delivery Market is witnessing remarkable expansion, fueled by several critical factors. One primary driver is the rising incidence of chronic illnesses such as diabetes, cancer, and heart diseases, creating a demand for sophisticated drug delivery methods that provide prolonged therapeutic effects and improve patient adherence. Moreover, the growing interest in personalized medicine is spurring advancements in controlled release technologies, which enable customized treatment plans that enhance drug effectiveness while reducing adverse effects. In addition, progress in polymer science and nanotechnology is leading to the development of advanced drug carriers that allow for greater precision and regulation of drug release rates. The increasing importance placed on geriatric care, coupled with a burgeoning elderly demographic, further propels market growth, as controlled release formulations offer practical solutions for managing multiple medications effectively. Additionally, ened investments in research and development by pharmaceutical enterprises are fostering the introduction of innovative drug delivery solutions that align with regulatory standards and fulfill market demands. Finally, the incorporation of digital health technologies, such as smart devices and monitoring tools, is improving the management and tracking of drug delivery systems, thus promoting enhanced patient involvement and adherence to prescribed therapies. Collectively, these elements highlight the robust growth potential of the controlled release drug delivery market.
Controlled Release Drug Delivery Market Restaining Factors:
Significant obstacles in the Controlled Release Drug Delivery Market comprise regulatory restrictions, elevated manufacturing expenses, and intricate formulation methodologies, all of which may impede innovation and limit accessibility.
The Controlled Release Drug Delivery Market is confronted by a number of factors that may hinder its expansion. One primary obstacle is the elevated manufacturing costs associated with the advanced technologies and materials necessary for developing controlled release formulations, which often require significant financial investments, thus restricting access for smaller pharmaceutical entities. Moreover, the stringent regulatory landscape can lead to extended approval timelines for novel controlled release products, causing delays that restrict their entry into the market. The formulation development process itself is quite intricate and demands specialized knowledge, posing a challenge particularly for emerging companies. Additionally, patient adherence represents a critical concern, as the success of controlled release systems is contingent upon correct usage; any deviations in patient compliance can result in less effective therapeutic results. The market also faces competition from other drug delivery systems, including more traditional approaches, potentially limiting the market share of controlled release technologies. Nonetheless, continuous advancements in materials science and technology, along with increased investment in research, are anticipated to foster innovation, ultimately improving the functionality and attractiveness of controlled release drug delivery systems. This progression not only aims to enhance patient outcomes but also offers promising opportunities for various stakeholders in the industry.
Segments of the Controlled Release Drug Delivery Market
By Technology:
- Coacervation
- Implants
- Micro Encapsulation
- Targeted Delivery
- Transdermal
- Wurster Technique
- Others (Liposomes, Microelectromechanical Technology)
By Release Mechanism:
- Partition Controlled Micro Reservoir Drug Delivery Systems
- Polymer Based Systems
- Drug Delivery Systems that are Feedback-regulated
- Drug Delivery Systems that are Activation-modulated
- Hydrodynamic Pressure Activated
- Osmotic Pressure Activated
- Magnetically Activated
- Vapor Pressure Activated
- Mechanically Activated
- Chemically Activated
- Hydrolysis Activated
- pH Activated
- Enzyme Activated
By Application:
- Injectable Metered Dose Inhalers
- Infusion Pumps
- Ocular and Transdermal Patches
- Oral Controlled-drug Delivery Systems
- Eluting Drug Stents
Regional Overview
North America
- US
- Canada
- Mexico
Europe
- Germany
- France
- U.K
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
Middle East and Africa
- Saudi Arabia
- UAE
- Rest of Middle East and Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America

