Clinical Trials Support Services Market Analysis and Insights:
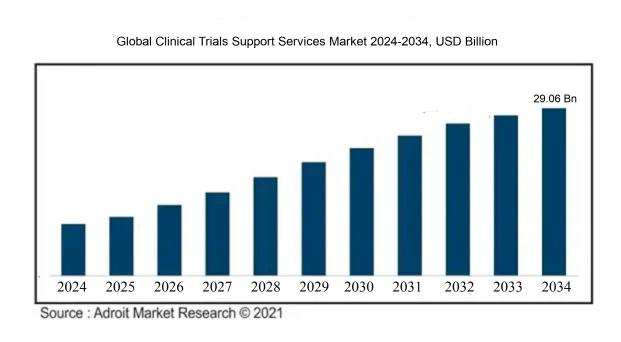
The market for clinical trials support services was valued at USD 26.03 billion in 2024, increased to USD 27.28 billion in 2025, and is expected to reach around USD 29.06 billion by 2034, indicating a robust compound annual growth rate (CAGR) of 8.10% from 2024 to 2034.
The expansion of the Clinical Trials Support Services Market is primarily influenced by the rising demand for groundbreaking treatments and the pressing need to streamline the drug development timeline. Increasing regulatory demands for expedited approvals, combined with ened investments in research and development from pharmaceutical companies, serve as additional catalysts for this market. The intricate nature of clinical trials, which often involve multi-national and multi-site collaborations, requires specialized support services to effectively manage logistics, data handling, and regulatory compliance. Moreover, technological advancements, such as artificial intelligence and big data analytics, significantly improve the efficiency of trial management and enhance patient recruitment techniques. The growing prevalence of chronic illnesses and an aging demographic further amplify the emphasis on clinical research, as pharmaceutical companies strive to meet evolving medical needs. Additionally, the rising partnerships between biotechnology firms and contract research organizations (CROs) are fostering collaborative resource allocation and expertise sharing, thereby driving market growth.
Clinical Trials Support Services Market Definition
Clinical Trials Support Services offer a comprehensive suite of solutions aimed at streamlining the design, implementation, and oversight of clinical studies. These services encompass the development of study protocols, adherence to regulatory standards, management of data, and strategies for patient enrollment, all contributing to the effective and ethical conduct of trials.
Clinical Trial Support Services are essential in the pharmaceutical development landscape, facilitating the effective oversight and implementation of clinical studies. These services offer critical resources such as regulatory expertise, strategies for patient recruitment, comprehensive data management, and monitoring to ensure the integrity of trials. By optimizing workflows and improving communication between all parties involved, they help to decrease both costs and timelines for the introduction of new treatments. Additionally, these services are instrumental in upholding compliance with strict regulatory standards, which enhances patient safety and the reliability of data. Their role is crucial in promoting medical innovation and responding to unmet healthcare needs through thorough scientific assessment.
Clinical Trials Support Services Market Segmental Analysis:

Insights On Service
Patient Recruitment Management
Patient Recruitment Management is expected to dominate the Global Clinical Trials Support Services Market due to the necessity of timely and effective recruitment of suitable patients for clinical trials. As the number of clinical trials has steadily increased, the need for efficient recruitment to ensure diverse patient populations and quicker results has never been more critical. Organizations focusing on developing advanced recruitment strategies, such as digital marketing, leveraging social media, and utilizing databases, are driving innovations in this area. Challenges such as participant retention and meeting enrollment targets highlight the importance of this service, positioning it as the most vital for successful clinical trial outcomes.
Clinical Trial Site Management
Clinical Trial Site Management plays a significant role in the Global Clinical Trials Support Services Market but is overshadowed by patient recruitment demand. This aspect focuses on the operations of trial sites, ensuring that facilities adhere to regulatory standards while maintaining effective communication between stakeholders. However, as recruitment challenges gain prominence, attention and resources have increasingly shifted towards optimizing patient recruitment, impacting the growth potential of site management activities.
Data Management
Data Management is crucial in maintaining the integrity and quality of clinical trial results, yet it faces challenges in gaining the same level of attention as patient recruitment. While the need for accurate data collection, storage, and analysis remains critical, advancements in digital technologies and automation have streamlined many data-related processes. Consequently, while important, this aspect does not attract the same priority or resources as recruiting patients effectively.
Administrative Staff
Administrative Staff services support the crucial logistical aspects of clinical trials but tend to operate behind the scenes. Their activities encompass managing budgets, coordinating schedules, and ensuring compliance with regulatory requirements. While essential for seamless operations, the visibility and impact of Administrative Staff roles are often less emphasized than those directly linked to patient engagement and recruitment, causing them to lag in market prominence.
IRB
Institutional Review Board (IRB) services ensure the ethical conduct of clinical trials and protect participants’ rights. However, although they are vitally important, their role is more regulatory than operational, resulting in less direct involvement in the day-to-day management of clinical trials. The need for regulatory oversight does not have the same level of market-driven demand as recruitment strategies, limiting the IRB's visibility compared to more proactive services related to patient involvement.
Others
The "Others" category encompasses various ancillary services that might support clinical trials but do not have a significant impact on market dynamics. These may include specialized consulting, training programs, or software tools not widely adopted. While these services can add value, they are generally less critical compared to more established services like patient recruitment management or data management. Consequently, they remain a minor focus in the competitive landscape of clinical trials support services.
Insights On Phase
Phase III
Phase III is expected to dominate the Global Clinical Trials Support Services Market primarily due to its critical role in establishing the efficacy and safety of new treatments before they receive regulatory approval. This phase involves large-scale testing among diverse populations which is essential for gaining the insights required for product licensing. As pharmaceutical companies increasingly invest in innovative therapies to meet the growing demand for effective treatment solutions, the complexity and size of Phase III trials are expanding. Consequently, this increases the need for robust support services encompassing patient monitoring, data management, regulatory compliance, and site management, making it the most significant phase in terms of market opportunities.
Phase I
Phase I focuses primarily on the safety and dosage of new drugs, typically involving a small number of participants. While it is crucial for identifying potential side effects, its market size is comparatively limited because of the reduced number of participants and trials. The focus here is on developing a foundational understanding of the drug's safety profile rather than broader efficacy, making its overall contribution to the clinical trials support services market less dominant than later stages.
Phase II
Phase II trials aim to evaluate the effectiveness of a drug, and while they are essential, they typically involve a moderate sample size and a narrower focus on specific conditions. This phase captures initial indications of efficacy and helps refine dosing recommendations, but like Phase I, its contribution to the broader market landscape is more limited compared to Phase III. As a result, while important, Phase II does not hold the same level of market influence or financial opportunity as later phases do.
Phase IV
Phase IV, or post-marketing surveillance, involves continual assessment of a drug's long-term effects after it has been approved for public use. Although this stage is crucial for monitoring safety and effectiveness in larger populations, it generally does not contribute significantly to the initial clinical trials support services market. Most funding and investments are directed towards the earlier phases, making Phase IV less dominant in the overall market context despite its importance in ongoing patient safety and drug performance assessments.
Insights On Sponsor
Pharmaceutical & Biopharmaceutical
The Pharmaceutical & Biopharmaceutical is expected to dominate the Global Clinical Trials Support Services Market due to the increasing investment in drug development and the growing prevalence of chronic diseases. This benefits from robust government support and favorable regulatory frameworks that facilitate clinical trials. Additionally, advancements in technology, such as artificial intelligence and machine learning, have streamlined the clinical trial processes, making them more efficient and cost-effective. The rising number of clinical research initiatives and the continuous demand for innovative therapies further enhance the market potential for pharmaceutical and biopharmaceutical sponsors, positioning them as the primary drivers in this industry.
Medical Devices
The Medical Devices category shows significant growth potential in the Global Clinical Trials Support Services Market. Increasing technological advancements in medical device manufacturing and design are driving the need for more clinical trials to ensure product efficacy and patient safety. Moreover, regulatory agencies are enforcing stricter compliance requirements, necessitating comprehensive trials to meet approval standards. As the medical device industry expands with new innovative products targeting various health ailments, the demand for clinical trial services to assess these devices continues to rise, making this a crucial player in the market.
Others
The Others category, which includes various sponsors such as academic institutions, non-profit organizations, and governmental bodies, contributes to the Global Clinical Trials Support Services Market, although it represents a smaller share than the dominant. Nonetheless, these entities play an important role in advancing research, especially in niche areas of medicine and public health. The growing collaboration between industry players and academic institutions enhances the research landscape, leading to unique clinical trials that explore emerging health trends and local diseases. Increased funding for research initiatives by governments and non-profits bolsters this 's participation in clinical trials, supporting broader health-related investigations.
Global Clinical Trials Support Services Market Regional Insights:
North America
North America is expected to dominate the Global Clinical Trials Support Services market due to several factors. The presence of numerous leading pharmaceutical and biotechnology companies, significant investment in research and development, and a highly regulated environment conducive to clinical trials contribute to this dominance. Additionally, the region benefits from advanced technological infrastructure, access to a large patient pool, and a well-established clinical research framework, making it an attractive hub for clinical trial operations. The popularity of outsourcing clinical trials to specialized service providers in North America also enhances its competitive edge. Furthermore, the continuous advancement in statistical methodologies and an increasing focus on patient-centric studies further solidify North America's leading position in this market.
Latin America
Latin America shows potential for growth within the Global Clinical Trials Support Services market, driven by cost-effective research opportunities and a rising focus on conducting trials in diverse populations. The region offers advantages such as faster patient recruitment and lower operational costs compared to North America and Europe. However, challenges related to regulatory hurdles and variability in compliance standards hinder its overall growth rate compared to more established region.
Asia Pacific
The Asia Pacific region is emerging as a notable player in the Global Clinical Trials Support Services market, propelled by its expanding patient demographics and cost advantages. Countries like India and China are witnessing a surge in clinical trial activities due to large patient populations and a growing emphasis on innovative research. While still in the growth phase, partnerships between local companies and global sponsors are strengthening the region's capabilities in clinical trial management, although concerns about regulatory complexities remain.
Europe
Europe holds a significant position in the Global Clinical Trials Support Services market, characterized by a mix of stringent regulatory standards and a robust healthcare infrastructure. Many European countries offer extensive clinical trial networks and access to a diverse patient population, which enhances the feasibility of conducting trials. However, the fragmented regulatory landscape across the region can be a challenge for trial sponsors looking for uniformity in execution processes, potentially impacting the speed and efficiency of trial setups.
Middle East & Africa
The Middle East & Africa region is gradually becoming involved in the Global Clinical Trials Support Services market, fueled by increased investment in healthcare infrastructure and growing participation in clinical research initiatives. While the region possesses unique advantages like diverse ethnic groups for trials, challenges such as limited access to modern research facilities, inconsistent regulatory environments, and financial constraints can impede faster growth. However, there is potential for expansion as regional governments and stakeholders recognize the importance of clinical research in improving healthcare standards.
Clinical Trials Support Services Competitive Landscape:
Prominent entities within the Global Clinical Trials Support Services sector are Contract Research Organizations (CROs) that oversee trial administration and data evaluation, alongside technology firms that deliver cutting-edge solutions for participant recruitment, oversight, and data gathering. Together, these organizations work synergistically to improve operational efficiency, ensure regulatory adherence, and boost patient involvement in clinical studies.
Prominent entities in the Clinical Trials Support Services sector encompass ICON plc, PRA Health Sciences, Covance Inc. (currently integrated with Labcorp), Syneos Health, Parexel International Corporation, Charles River Laboratories, Medpace Holdings, Inc., WuXi AppTec, QuintilesIMS (now under IQVIA), PPD, Inc., Aptiv Solutions, Biorasi, Clinipace, Worldwide Clinical Trials, and BioClinica.
Global Clinical Trials Support Services COVID-19 Impact and Market Status:
The Global Clinical Trials Support Services market experienced significant disruptions due to the Covid-19 pandemic, which resulted in postponed timelines and alterations to trial protocols. Concurrently, this crisis prompted a faster shift towards decentralized trial frameworks.
The COVID-19 pandemic had a profound effect on the Clinical Trials Support Services Market, presenting both hurdles and new possibilities. At the outset, numerous clinical trials encountered delays and suspensions due to restrictions on patient enrollment and access to study sites, which in turn influenced schedules and financial considerations. Conversely, the crisis acted as a catalyst for the rapid adoption of cutting-edge technologies like telehealth and decentralized clinical trial approaches, which improved patient participation and the efficiency of data gathering. The urgent need for vaccine development and rapid diagnostic testing ened the demand for support services, prompting companies to adjust and enhance their operational strategies. Moreover, regulatory agencies responded by providing greater flexibility, expediting approval processes for COVID-19-related trials. The transition to remote monitoring and virtual trial formats emphasized the growing necessity for comprehensive support services. Ultimately, although the pandemic disrupted conventional clinical trial practices, it initiated significant methodological changes, demonstrating the resilience and adaptability inherent within the clinical research domain.
Latest Trends and Innovation in The Global Clinical Trials Support Services Market:
- In August 2023, Covance, part of Labcorp Drug Development, announced a strategic acquisition of the clinical trial management software company, Medidata Solutions, to enhance its technology offerings in clinical operations.
- In July 2023, ICON plc completed its acquisition of PRA Health Sciences, a move aimed at expanding ICON's global reach and service capabilities in the clinical trials support sector.
- In January 2023, Syneos Health launched its new Patient Experience Solutions, utilizing advanced analytics and digital technology to improve patient engagement and retention in clinical trials.
- In September 2022, Quest Diagnostics acquired the clinical trial laboratory services of the healthcare provider, BioReference Laboratories, enhancing its laboratory capabilities in supporting clinical research.
- In June 2022, Charles River Laboratories entered into a strategic partnership with EBR Systems to co-develop and deliver advanced cardiac monitoring solutions tailored for clinical trials.
- In April 2022, Medpace Holdings, Inc. announced the expansion of its oncology clinical trial services through the establishment of a specialized Oncology Research Center, enhancing its position in the oncology research market.
- In March 2022, PPD, a part of Thermo Fisher Scientific, launched an innovative software platform that integrates real-time data analytics to streamline clinical trial operations and improve decision-making.
- In February 2022, Parexel International announced a partnership with Google Cloud to enhance its data analytics and artificial intelligence capabilities, aiming to accelerate clinical trial timelines and improve patient outcomes.
- In December 2021, WuXi AppTec acquired the clinical research organization, XenoBiotic Laboratories, to bolster its preclinical and clinical trial capabilities in drug development.
- In November 2021, BioClinica announced the expansion of its imaging services in clinical trials by adding advanced AI-driven technology that aims to enhance the efficiency of imaging data collection and analysis.
Clinical Trials Support Services Market Growth Factors:
The expansion of the Clinical Trials Support Services Market is propelled by technological innovations, an upsurge in funding for pharmaceutical development, and a growing need for effective management solutions in clinical trials.
The Clinical Trials Support Services Market is witnessing considerable expansion, driven by a variety of influential elements. Primarily, the intricacies associated with clinical trials have escalated due to ened patient expectations and strict regulatory frameworks, resulting in an increased need for specialized support solutions. Furthermore, the rising incidence of chronic illnesses and the urgency for accelerated drug development highlight the demand for effective trial management systems. Technological advancements, particularly in areas such as data analytics, artificial intelligence, and digital platforms, are optimizing the clinical trial workflow, facilitating patient recruitment, and enhancing the overall efficacy of trials.
Additionally, the trend towards decentralized and adaptive trial methodologies is transforming the sector by enabling more versatile approaches and fostering greater patient involvement. The pharmaceutical and biotechnology industries are also significantly channeling resources into outsourcing clinical trial operations to expert service providers, thereby contributing to market growth. The focus on personalized medicine and precision therapies is sparking an increase in specialized clinical trials, further broadening the scope for support services. Collectively, these dynamics are driving the advancement of the Clinical Trials Support Services Market as stakeholders seek efficient, compliant, and patient-focused solutions in a progressively competitive landscape.
Clinical Trials Support Services Market Restaining Factors:
The Clinical Trials Support Services Market faces several significant obstacles, including intricate regulatory frameworks, elevated operational expenses, and difficulties in attracting and maintaining patient participation.
The market for Clinical Trials Support Services encounters multiple challenges that may hinder its development. A major obstacle is the intricate nature of regulatory frameworks, as differing regulations and compliance standards in various nations can result in delays and escalated operational expenses for service providers. Furthermore, the substantial financial demands of clinical trials, particularly in areas like patient recruitment and retention, can discourage smaller biotech firms from initiating new research, constraining market growth. Additionally, there is frequently a lack of qualified personnel in clinical trial management, which can impede the effective execution of trials. The rising emphasis on data privacy and security introduces further complications, as companies must comply with stringent regulations while handling sensitive patient data. The swift evolution of technology also contributes to the difficulties, requiring ongoing adjustments and investments to maintain a competitive edge. Nevertheless, in spite of these obstacles, the market holds potential for growth, fueled by increasing investments in research and development, the rise of novel therapeutic strategies, and a growing focus on patient-centered trial methodologies. With continued efforts to mitigate these issues, the Clinical Trials Support Services Market is set to develop and prosper in the future, presenting considerable opportunities for various stakeholders.
Key Segments of the Clinical Trials Support Services Market
By Service:
- Clinical Trial Site Management
- Data Management
- Patient Recruitment Management
- Administrative Staff
- IRB (Institutional Review Board)
- Others
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
By Sponsor:
- Pharmaceutical & Biopharmaceutical
- Medical Devices
- Others
Regional Overview
North America
- US
- Canada
- Mexico
Europe
- Germany
- France
- U.K
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
Middle East and Africa
- Saudi Arabia
- UAE
- Rest of Middle East and Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America

