Cell and Gene Therapy Clinical Trial Market Analysis and Insights:
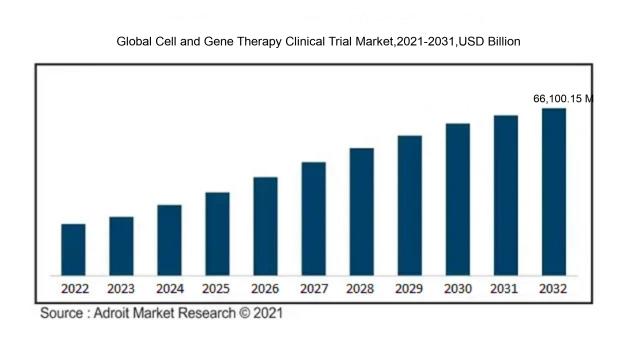
In 2023, the size of the worldwide Cell and Gene Therapy Clinical Trial market was US$ 10,405 million. Adroit Market Research projects that the market will increase at a compound annual growth rate (CAGR) of 24.8% from 2024 to 2032, reaching US$ 66,100.15 million.
The market for clinical trials in cell and gene therapy is influenced by several pivotal factors. A notable increase in genetic disorders and chronic illnesses has created a demand for groundbreaking treatment options. Advancements in biotechnology and genetic modification techniques are paving the way for new therapies, thus attracting significant investments from both public and private entities. Support from regulatory bodies like the FDA and EMA, particularly through accelerated approval processes for gene and cell therapies, streamlines the initiation of trials. Additionally, the rising rates of cancer and other severe health conditions are driving the need for effective treatment solutions. Increased patient awareness and the push for personalized medicine further encourage participation in clinical trials. Collaborations between pharmaceutical companies, academic research institutions, and biotech firms stimulate innovation and speed up the development of new therapies. Lastly, continuous funding for research and technological progress in precision medicine are contributors to the evolving landscape of clinical trials for cell and gene therapies.
Cell and Gene Therapy Clinical TrialMarket Definition
A clinical trial focused on cell and gene therapy is a meticulously structured investigation aimed at assessing the safety and effectiveness of interventions that alter or substitute genes or cells to tackle a range of medical conditions. Such trials play a crucial role in exploring the therapeutic possibilities and formulating guidelines for groundbreaking treatments.
Clinical trials focused on cell and gene therapies play an essential role in developing groundbreaking treatments for diseases that have long been considered untreatable. These trials are instrumental in evaluating both the safety and effectiveness of therapeutic approaches that involve genetic alterations or cellular interventions aimed at correcting or alleviating genetic disorders, cancers, and other complex health issues. By contributing to the expansion of scientific knowledge and establishing regulatory guidelines, these investigations not only improve patient care but also enhance the prospects for personalized medicine. The successful outcome of these trials could pave the way for revolutionary therapies that markedly enhance patient outcomes and overall quality of life, representing a noteworthy leap forward in contemporary medicine.
Cell and Gene Therapy Clinical Trial Market Segmental Analysis:

Insights On Phase
Phase I
Phase I is anticipated to dominate the Global Cell and Gene Therapy Clinical Trial Market primarily due to its essential role in assessing safety and dosage for new treatments. This phase acts as the first step in evaluating therapeutic interventions on humans, allowing researchers to identify potential side effects and optimal dosages for subsequent trial phases. The increasing focus on personalized medicine and innovative therapies requiring initial safety assessments has created a substantial demand for Phase I trials specifically in the cell and gene therapy sectors. Moreover, funding for early-stage trials is expanding, further strengthening the position of Phase I as a critical component in clinical research.
Phase II
Phase II is crucial for confirming the efficacy of a treatment following initial safety assessments performed in Phase I. Trials in this stage not only involve larger groups of patients but also help determine the optimal dose and gather more comprehensive data on side effects. The growing emphasis on targeted therapies within gene and cell treatments ensures that Phase II is vital to the clinical workflow, but it tends to attract fewer resources compared to the earlier phase because of its higher costs and complexities in trial design. Although it plays a significant role, it remains secondary compared to the prominence achieved by Phase I trials.
Phase III
Phase III trials play a decisive role in obtaining regulatory approval for new therapies, testing the effectiveness of treatments against standard therapies in large populations. While they serve a critical purpose in the clinical trial landscape, these trials are generally more resource-intensive and costly. Their timeline extends significantly, often affecting their overall prevalence in the market. With increased interest in gene and cell therapy innovation, the number of Phase III trials has been on the rise, but they still follow Phase I in terms of market impact due to the extensive preparatory work required beforehand.
Insights On Indication
Oncology
Oncology is expected to dominate the Global Cell and Gene Therapy Clinical Trial Market due to the substantial prevalence of cancer and the increasing demand for innovative treatment options. With advancements in gene editing technologies and the growing understanding of the tumor microenvironment, oncology-focused therapies are garnering significant attention. Clinical trials in this area are often well-funded and have a robust pipeline as pharmaceutical companies prioritize expanding their portfolios in cancer treatment. The ability of gene therapies to target and modify specific cancerous cells is driving a significant shift toward precision medicine, positioning oncology as the leading area within the market.
Cardiology
Cardiology is witnessing a rising interest in cell and gene therapies, especially with conditions like heart failure and ischaemic heart diseases becoming prevalent. The potential for regenerative therapies to repair cardiac tissue and improve heart function presents a compelling proposition for researchers and investors alike. A growing body of research is focused on stem cell-based therapies to enhance heart regeneration and restore normal function, thus fostering an optimistic outlook for this sector in the clinical trial market.
CNS (Central Nervous System)
The Central Nervous System is increasingly recognized for its critical health challenges, including neurodegenerative diseases and brain injuries. Ongoing clinical trials aim to explore gene therapies that target conditions like Alzheimer’s and Parkinson’s disease. Innovations in delivery methods and a rising emphasis on neurological disorders are attracting significant investment, promising a more pronounced presence in the future of clinical trials within this therapeutic area.
Musculoskeletal
Musculoskeletal disorders have gained attention due to their high incidence rates and the potential for innovative therapies to promote tissue regeneration and repair. Clinical trials focusing on gene therapy for osteoarthritis and other musculoskeletal conditions are growing, driven by the desire to improve patient outcomes. Moreover, advancements in cartilage regeneration techniques and the use of stem cells in musculoskeletal applications are opening new avenues for research, further enhancing the sector’s attractiveness.
Infectious Diseases
Infectious diseases, including viral infections and chronic diseases like HIV, are significantly impacting global health. The potential of gene therapy to develop vaccines and treat stubborn infections is garnering interest in clinical trials. The response to emerging infectious threats, like COVID-19, has accelerated research initiatives and funding aimed at innovative therapeutic solutions in this field, marking it as an important.
Dermatology
Dermatology is increasingly exploring gene and cell therapies aimed at conditions such as psoriasis, eczema, and genetic skin disorders. The focus on innovative approaches to treat and manage chronic skin conditions has led to a number of promising clinical trials. The potential for gene transfer technologies to address underlying genetic defects in skin disorders adds value in this, driving growth and interest.
Endocrine
Endocrine disorders, including diabetes and hormonal imbalances, are significant health challenges globally. Cell and gene therapies that aim to restore normal endocrine function or enhance insulin production are currently under investigation. Interest in addressing metabolic disorders through these innovative approaches offers a hopeful outlook for clinical development within this critical therapeutic area.
Metabolic
The metabolic disorders sector is gaining traction as awareness of the burden of conditions such as obesity and metabolic syndrome rises. Clinical trials are increasingly focusing on gene therapies that can influence metabolic pathways and potentially reverse the effects of these disorders. As researchers continue to explore how genetic approaches can enhance metabolic regulation, this area is poised for growth.
Genetic
The genetic disorders category remains a crucial focus within the cell and gene therapy landscape, primarily investigating inherited conditions like cystic fibrosis and muscular dystrophy. The potential for gene editing technologies, including CRISPR, to directly alter pathogenic genes lays the groundwork for transformative therapies. This is particularly appealing due to consistent advancements and breakthroughs in genetic research.
Immunology & Inflammation
Immunology and inflammation are drawing attention, especially concerning autoimmune diseases and chronic inflammatory conditions. Therapeutic approaches that aim to modify immune responses through gene therapy are gaining traction. As the understanding of immune pathways deepens, it enables targeted gene therapies to be developed, marking this area as one with significant growth potential.
Ophthalmology
Ophthalmology stands out for its focus on genetic conditions affecting vision, such as retinitis pigmentosa and age-related macular degeneration. Gene therapies are becoming a breakthrough approach in managing these disorders, leading to renewed interest in clinical trials for innovative treatments. Research aimed at restoring sight through gene therapy positions this therapeutic area on a promising growth trajectory.
Hematology
The hematology field is experiencing significant advancements due to gene therapies targeting blood disorders like hemophilia and sickle cell disease. The ability to correct genetic defects and provide long-term solutions for patients creates substantial opportunity for clinical trials. The enhanced understanding of hematopoietic stem cell manipulation is driving interest and investment in this sector, showing strong potential for growth.
Gastroenterology
Gastroenterology is starting to harness the potential of cell and gene therapies aimed at inflammatory bowel diseases and other gastrointestinal disorders. As the complexity of these conditions becomes clearer, innovative therapeutic strategies are being explored via clinical trials. The focus on genetic and cellular interventions indicates a slowly emerging area with future growth possibilities in the clinical trial market.
Global Cell and Gene Therapy Clinical Trial Market Regional Insights:
North America
North America is projected to dominate the Global Cell and Gene Therapy Clinical Trial market, primarily driven by its advanced healthcare infrastructure, significant investment in biopharmaceutical research, and supportive regulatory environment. The region boasts numerous leading biopharmaceutical companies and research institutions that are at the forefront of developing innovative therapies. High levels of funding and a robust venture capital scene further enhance research and development activities. Additionally, collaborations among academia, industry, and regulatory bodies expedites clinical trial processes, enabling quicker translation of findings into therapeutic solutions. With a strong emphasis on precision medicine, North America remains a vital hub for cell and gene therapy advancements.
Latin America
Latin America has shown potential for growth in the cell and gene therapy clinical trial market, driven by increasing investment in healthcare and development of biopharmaceutical sectors. Countries such as Brazil and Mexico are recognizing the importance of clinical research as a means to bolster local healthcare solutions. Challenges such as regulatory hurdles and limited infrastructure still persist, but emerging partnerships among local and international entities are fostering a more conducive environment for clinical trials. As the region continues to strengthen its research capacity, it may emerge as a favorable site for future cell and gene therapies.
Asia Pacific
Asia Pacific is steadily gaining traction in the cell and gene therapy clinical trial market, largely due to the region’s rapidly evolving healthcare landscape and growing patient population. Countries like China, Japan, and India have made considerable advancements in biotechnology research, spurring numerous clinical trials. Government initiatives and investment in healthcare infrastructure contribute positively, while a rising awareness of personalized medicine drives demand for innovative therapies. However, varying regulatory frameworks and the need for standardized practices present challenges that will need to be addressed for growth to materialize effectively.
Europe
Europe possesses a robust regulatory framework and rich scientific expertise, positioning it as a competitive player in the cell and gene therapy clinical trial arena. The European Union’s commitment to investing in innovative healthcare solutions and biotechnology as part of its agenda aids in advancing clinical trials. Countries like Germany, the UK, and France lead in terms of research output, while multinational collaborations enhance the region's capabilities. Nonetheless, the market is fragmented, and navigating differing national regulations can pose challenges, ultimately impacting the speed at which therapies can reach the market.
Middle East & Africa
The Middle East & Africa region remains nascent in the cell and gene therapy clinical trial market, with ongoing investments in healthcare and research needed to develop a more solid foundation. While certain countries are investing in advanced technologies and boosting their attractiveness for clinical trials, significant disparities in infrastructure, regulatory frameworks, and healthcare access limit the region’s immediate potential. Nevertheless, as regional governments begin to recognize the importance of biotechnology, there may be promising growth opportunities in the future for localized trials and increased participation in global studies.
Cell and Gene Therapy Clinical Trial Market Competitive Landscape:
Prominent participants in the international market for clinical trials in cell and gene therapy encompass biopharmaceutical firms responsible for the development and production of therapeutic solutions, alongside regulatory bodies that monitor trial protocols to guarantee safety and effectiveness. Partnerships between academic institutions and contract research organizations (CROs) play a crucial role in fostering innovation and coordinating the logistics of trials.
Prominent entities in the clinical trial arena for cell and gene therapies encompass Novartis AG, Gilead Sciences, Inc., Bristol-Myers Squibb Company, Spark Therapeutics, Inc., bluebird bio, Inc., Regeneron Pharmaceuticals, Inc., Amgen Inc., Roche Holding AG, Sanofi S.A., and Celgene Corporation (which operates under Bristol-Myers Squibb). Other notable contributors include Merck & Co., Inc., Kite Pharma, Inc. (a Gilead subsidiary), CRISPR Therapeutics AG, Editas Medicine, Inc., Intellia Therapeutics, Inc., and 23andMe, Inc. Furthermore, companies such as Cellectis S.A., Anjarium Biosciences AG, Orchard Therapeutics plc, and AVROBIO, Inc. are also influential within this rapidly evolving sector. These organizations are dedicated to pioneering therapeutic advancements and executing clinical trials that propel the development of cell and gene therapies.
Global Cell and Gene Therapy Clinical Trial Market COVID-19 Impact and Market Status:
The Covid-19 pandemic caused considerable interruptions to clinical trials for cell and gene therapies worldwide, resulting in postponements, alterations in recruitment approaches, and a greater reliance on telemedicine to ensure continued patient involvement and data gathering.
The COVID-19 pandemic had a profound effect on the clinical trial landscape for cell and gene therapies, causing significant delays and interruptions during its onset. Restrictions such as lockdowns and limitations on hospital access impeded the recruitment of participants and follow-up processes, which urged researchers to modify trial protocols. This included the incorporation of remote monitoring and telehealth solutions to ensure continuity. Despite these early setbacks, the crisis spurred an uptick in investments towards innovative therapeutic strategies, underscoring the critical need for advanced treatments targeting genetic conditions and chronic illnesses. In response to the urgency, regulatory bodies implemented more adaptable guidelines, which streamlined the approval pathways for essential therapies. As a result, although the pandemic initially posed challenges in terms of timelines and participant enrollment, it ultimately fostered a robust recovery and an increase in funding for cell and gene therapy initiatives in the following years. This complex interplay reflects a transformed environment for clinical trials, showcasing both resilience and ingenuity amid global challenges.
Latest Trends and Innovation in The Global Cell and Gene Therapy Clinical Trial Market:
- In July 2023, Vertex Pharmaceuticals announced its acquisition of Semma Therapeutics for $950 million, focusing on advancing gene-editing therapies for diabetes, particularly using stem cell-derived therapeutic approaches.
- In June 2023, Novartis acquired gymnema biotech company, Gene Therapy Company, to bolster its gene therapy pipeline, specifically targeting diseases related to metabolic disorders.
- In September 2023, Gilead Sciences initiated a Phase 1 clinical trial for its gene therapy product aimed at treating sickle cell disease, marking an important advance in the application of CRISPR technology in therapeutic contexts.
- In August 2023, Bluebird Bio received FDA approval for its gene therapy for cerebral adrenoleukodystrophy (CALD), enabling treatment options for patients with this rare genetic disorder, showcasing a significant milestone in the cell and gene therapy sector.
- In April 2023, Amgen partnered with Regeneron Pharmaceuticals to explore the potential of bispecific T-cell engagers in cell therapy, emphasizing the role of advanced therapeutic modalities in oncology.
- In March 2023, CRISPR Therapeutics and Vertex Pharmaceuticals announced positive interim results from their ongoing CLIMB-121 trial of CTX001, a CRISPR gene-editing therapy targeting beta-thalassemia and sickle cell disease.
- In May 2023, Pfizer and Sangamo Therapeutics expanded their collaboration to include new gene therapy projects, aiming to accelerate the development of novel treatments for genetic diseases.
- In February 2023, Tara Therapeutics commenced a pivotal Phase 3 trial for its gene therapy designed to treat X-linked severe combined immunodeficiency (X-SCID), indicating a growing focus on pediatric gene therapies.
- In October 2023, the FDA granted breakthrough therapy designation to Editas Medicine for its gene-editing therapy targeting Leber congenital amaurosis, a rare genetic eye disorder, reflecting the regulatory body’s support for innovative therapy developments.
- In January 2023, AstraZeneca and Moderna announced a strategic collaboration to develop mRNA-based therapeutics, combining AstraZeneca’s expertise in oncology with Moderna’s mRNA technology for potential cell therapy applications.
Cell and Gene Therapy Clinical Trial Market Growth Factors:
The market for clinical trials in cell and gene therapies is currently witnessing substantial expansion, propelled by technological advancements, rising investments in R&D, and an increased appetite for cutting-edge therapeutic solutions.
The market for Cell and Gene Therapy Clinical Trials is witnessing remarkable expansion driven by multiple factors. Primarily, the increasing incidence of genetic disorders and chronic illnesses is fueling the need for groundbreaking therapeutic alternatives. Innovations in biotechnology and genetic modification have resulted in safer and more effective treatment options, thereby enhancing the viability of cell and gene therapies. Additionally, the rise in investments from both the private and public sectors, alongside the development of supportive regulatory policies, has created a favorable landscape for conducting clinical trials. Collaborative efforts between academic institutions and biotech firms are further propelling research advancements and improving trial methodologies. Moreover, ened awareness among healthcare practitioners and patients regarding the advantages of these therapies is leading to greater participation in clinical studies. Positive outcomes from recent prominent trials have generated a wave of optimism about the effectiveness of these treatments, attracting increased financial support and interest. Finally, a growing array of therapies aimed at rare and intricate diseases, combined with advancements in manufacturing techniques, is likely to facilitate ongoing growth in the market. Collectively, these elements are substantially enhancing the dynamics of the Cell and Gene Therapy Clinical Trial Market.
Cell and Gene Therapy Clinical Trial Market Restaining Factors:
Regulatory barriers and elevated manufacturing expenses present considerable obstacles to the expansion of the clinical trial market for cell and gene therapies.
The landscape of the Cell and Gene Therapy Clinical Trial Market is influenced by various challenges that may impede its expansion. One of the primary obstacles is the substantial financial burden associated with the research, development, and production of these cutting-edge therapies, which can deter both pharmaceutical companies and investors. Additionally, the intricate nature of regulatory processes can lead to delays in obtaining necessary approvals, as authorities demand thorough data on safety and effectiveness, resulting in lengthened timelines and higher costs.
Moreover, the lack of adequately trained professionals within this niche sector poses significant hurdles in executing trials efficiently, which may ultimately disrupt project schedules. Ethical dilemmas surrounding genetic alterations and the uncertain long-term consequences of gene therapies could also negatively affect public perception and acceptance. Compounding these issues is the presence of established traditional therapies, which may restrict the adoption and integration of cell and gene therapies, particularly in markets with existing treatment infrastructures.
Nonetheless, there is a sense of optimism within the market due to continual technological innovations, increased financial investments, and a growing understanding of the advantages associated with personalized medicine. As research evolves and regulatory landscapes change, the Cell and Gene Therapy Clinical Trial Market is anticipated to undergo substantial transformation, offering the potential for groundbreaking treatment avenues for numerous diseases.
Segments of the Cell and Gene Therapy Clinical Trial Market
By Phase
- Phase I
- Phase II
- Phase III
By Indication
- Oncology
- Cardiology
- CNS
- Musculoskeletal
- Infectious Diseases
- Dermatology
- Endocrine
- Metabolic
- Genetic
- Immunology & Inflammation
- Ophthalmology
- Hematology
- Gastroenterology
- Other Indications
Regional Overview
North America
- US
- Canada
- Mexico
Europe
- Germany
- France
- U.K
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
Middle East and Africa
- Saudi Arabia
- UAE
- Rest of Middle East and Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America

